Pharmacological treatment of polycystic ovary syndrome – health and quality of life in the short and long term
A systematic review including health economic and ethical aspects
Main message
Polycystic ovary syndrome (PCOS) is a common condition in women, caused by hormonal variations. Pharmacological treatments for PCOS can have some positive short-term effects, but there are major evidence gaps about their long-term effectiveness and safety. This report investigates the effects of combined oral contraceptive pills (COCPs), antiandrogen drugs, metformin and glucagon-like peptide-1 analogues (GLP-1 analogues) in individuals with PCOS.
Conclusions
- COCPs and metformin may improve menstrual frequency and regularity. The evidence for GLP-1 analogues and antiandrogens is insufficient to assess their effect on menstruation.
- Hirsutism, excessive growth of hair on the body or face, is a common symptom in women with PCOS. COCPs that contain cyproterone acetate (CPA) may have a slightly better effect on hirsutism compared to regular COCPs. For other comparisons between types of COCPs, or COCPs vs. no treatment, evidence is insufficient. Antiandrogens, whether alone or as add-ons, do not seem effective, but the evidence base is limited.
- Metformin and GLP-1 analogues can lead to some weight loss in individuals with overweight or obesity. Evidence is insufficient for weight effects of COCPs and antiandrogens. Metformin provides modest improvement in markers for glucose and insulin regulation but shows no effect on blood lipids.
- For all the evaluated drugs, the evidence is insufficient to assess their effects on quality of life.
- There is also insufficient evidence to determine the long-term health effects of these treatments for PCOS. This is due to a lack of long-term studies.
Aim
The purpose of this systematic review was to evaluate the efficacy, safety, short and long-term health impacts, health-economic aspects, and ethical considerations of pharmacological treatments currently used – or potentially applicable – for individuals diagnosed with PCOS.
Background
PCOS is a common hormonal disorder in women of reproductive age, affecting about 12% of adult women. It is characterized by elevated androgen levels, irregular or absent menstruation, and multiple ovarian follicles. PCOS is also associated with insulin resistance, overweight or obesity, infertility, and increased risk for type 2 diabetes, cardiovascular disease, endometrial cancer, and mental health issues such as anxiety and depression.
Diagnosis typically requires at least two out of three criteria: hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology. The condition presents differently across individuals and over the lifespan.
There is no cure for PCOS; treatments aim to manage symptoms. Lifestyle changes are first-line therapy, especially for individuals with overweight or obesity. Pharmacological treatments – often off-label – include COCPs, metformin and antiandrogens. GLP-1 analogues can be used in patients with type 2 diabetes or obesity.
The healthcare system often addresses symptoms in isolation, which may lead to fragmented care. A holistic, multidisciplinary approach is increasingly recommended. Updated international guidelines were released in 2023, but Sweden lacks a national care program for PCOS, motivating this comprehensive evidence review.
Method
We conducted a systematic review following the standard methods used by SBU and reported it in accordance with the PRISMA statement. The protocol was registered in Prospero (CRD42024535096). The review included randomized controlled trials (RCTs) and observational studies (for long-term outcomes), used meta-analyses when possible, and applied the GRADE system to assess certainty of evidence. Additional literature searches were conducted for health economic studies and a chapter on ethical aspects was included.
Inclusion criteria
- Population: Women of all ages with PCOS diagnosed per Rotterdam, NIH, or AES criteria. Studies on pregnant women were excluded or if pregnant patients were included, they had to be reported separately. In register-based studies diagnostic code equivalent with a clinical diagnosis of PCOS was required.
- Intervention: COCPs, antiandrogen drugs, metformin, GLP-1 analogues.
- Control: Placebo, no treatment, lifestyle intervention, or comparison with other drug/drugs in one or several of the intervention categories.
- Outcomes: Menstrual regularity, hirsutism, body mass index (BMI), waist-hip ratio (WHR), insulin/glucose markers (fasting insulin, fasting glucose and HOMA-IR), blood lipids (triglycerides (TG), LDL (low density lipoprotein) cholesterol, psychological outcomes, quality of life, adverse effects, long-term outcomes (e.g. diabetes, CVD, cancer, mortality).
- Study design: RCTs for short-term effects; RCTs and cohort/case-control studies (≥100 participants) for long-term outcomes.
- Language: English, Swedish, Norwegian, or Danish.
Databases searched: MEDLINE (Ovid), EMBASE, Cochrane Library, CINAHL, PsycINFO. Last literature search was performed in Oct 2024. - Health economic assessment: for GLP-1-analogues the literature was screened for health economic assessments for cost and cost-effectiveness.
- Patient involvement: Input from a patient representative helped shape outcome priorities and ethical discussions.
Results
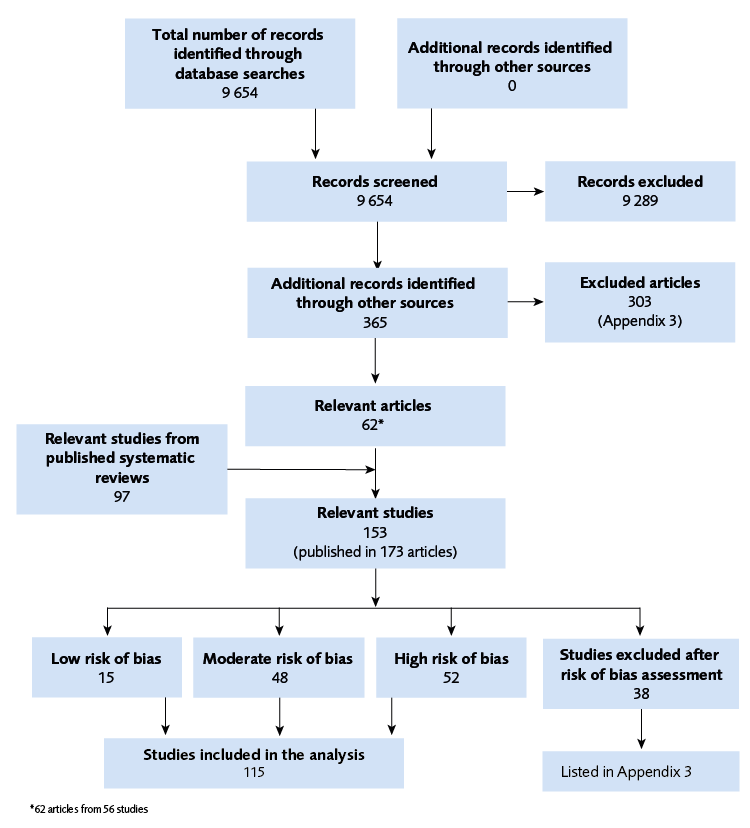
A total of 9654 abstracts were identified by the literature search, 365 were read in full text and 62 articles (56 studies) were eligible. To these another 97 eligible studies were added from five previously published systematic reviews, yielding a total of 153 relevant studies. A further 38 were excluded as they were determined to have a critical risk of bias, leaving 115 studies included in the analyses, see Figure 1.
Combined oral contraceptive pills
When COCPs were compared to no treatment, menstrual regularity and frequency was improved, with low certainty (⊕⊕◯◯). For all other outcomes the level of certainty was very low (⊕◯◯◯). No studies on this comparison were added in the updated search for this report.
In the comparison between different types of COCPs, one study was added in this report, compared to the previously published systematic review. Most comparisons had a result with very low certainty of evidence (⊕◯◯◯). However, patients treated with contraceptive pills containing cyproterone acetate and ethyl estradiol had a lower BMI and reduced levels of hirsutism after treatment compared to patients treated with other types of COCPs, both results with low certainty (⊕⊕◯◯). For fasting insulin and the same comparison, no difference was observed (⊕⊕◯◯).
Antiandrogen drugs
In the comparison of different types of antiandrogen drugs and other treatments or no treatment, there was no added effect of antiandrogen drugs regarding hirsutism, fasting glucose or triglycerides, with low certainty of evidence (⊕⊕◯◯). For all other outcomes the level of certainty was very low (⊕◯◯◯). No studies were added in the updated search for this report, but we performed additional meta-analyses.
Metformin and combined oral contraceptive pills
In comparisons between COCPs alone and in combination with metformin, no differences were found in BMI (⊕⊕◯◯), WHR (⊕⊕⊕◯), or hirsutism (⊕⊕⊕◯). Fasting insulin and HOMA-IR improved slightly more with combination treatment (⊕⊕◯◯) while fasting glucose showed no difference (⊕⊕◯◯). Lipid levels also showed no differences between groups (low to moderate certainty).
When metformin plus COCPs were compared to metformin alone, most of our prioritized outcomes had very low certainty (⊕◯◯◯). No differences were found for fasting insulin or LDL cholesterol (⊕⊕◯◯ ).
No studies were added in the updated search for this report.
Metformin
Three studies were found in the updated search for this report and added to the 29 studies included in the original systematic review. New meta-analyses were performed comparing studies were metformin with or without lifestyle interventions were compared to no treatment, placebo or lifestyle interventions only.
| Outcome | Meta-analysis (patients; studies) * |
Results (favors; point estimate; 95% confidence interval) |
GRADE |
|---|---|---|---|
| BMI | 312 patients 6 studies |
Favors GLP-1 analogues –1.38 (–2.39 to –0.38) |
⊕⊕◯◯ |
| WHR | 192 patients 3 studies |
No difference –0.01 (–0.10 to 0.08) |
⊕◯◯◯ |
| Fasting glucose | 319 patients 5 studies |
No difference –0.10 (–0.46 to 0.26) |
⊕◯◯◯ |
| Fasting insulin | 192 patients 3 studies |
No difference 9.72 (–30.14 to 49.59) |
⊕◯◯◯ |
| HOMA-IR | 260 patients 4 studies |
No difference –0.28 (–2.36 to 1.80) |
⊕◯◯◯ |
| LDL cholesterol | 260 patients 5 studies |
No difference 0.01 (–0.04 to 0.05) |
⊕⊕⊕◯ |
| Triglycerides | 127 patients 2 studies |
No difference –0.05 (–0.26 to 0.17) |
⊕⊕◯◯ |
| Hirsutism | 72 patients 1 study** |
No difference |
⊕◯◯◯ |
| Menstruation | 250 patients 5 studies |
Favors GLP-1 analogues (synthesis without meta-analysis) |
⊕◯◯◯ |
| Abbreviations: BMI= body mass index; GLP-1 analogues= glucagone like peptide 1 analogues; LDL= low density lipoprotein; WHR= waist-hip-ratio *In the original report, studies that reported results that could not be included in a meta-analysis were reported narratively and reported in the summary of findings table ** Result in study only reported narratively |
|||
Long-term outcomes of pharmacological treatments
The literature search identified five register-based studies that filled the inclusion criteria. Also, twenty randomized controlled studies on COCPs, antiandrogen drugs or metformin had a follow up period of 12 months or longer. No long-term studies on the effects or side effects of GLP-1-analogues were identified.
The scientific evidence was insufficient to assess the impact of these pharmacological treatments on important health outcomes for patients with PCOS, such as diabetes, cardiovascular disease, mental health disorders, or endometrial changes.
Treatment with COCPs containing CPA likely improves hirsutism more effectively than standard combined contraceptives after 12 months (⊕⊕◯◯). However, this benefit must be weighed against the known risks associated with CPA. For all other outcomes assessed in RCTs, such as menstrual regularity, hirsutism, weight related or metabolic outcomes, no conclusions could be drawn regarding long-term health and treatment with COCPs, antiandrogen drugs and metformin.
Health Economic Assessment
The health economic review focused on GLP-1 analogues due to their higher cost level compared to the other pharmacological treatments evaluated in this report. No cost-effectiveness studies specific to PCOS were found for GLP-1 analogues. The assessment also mapped Swedish reimbursement status for all the drugs included in this report.
Ethical and societal aspects
PCOS is a common condition were patients in Sweden have unequal access to care across regions and care levels, often leading to fragmented treatment. Many medications are used off-label without strong evidence, raising ethical concerns, especially regarding the long-term benefit of treatments. There may be stigma associated to certain aspects of PCOS, such as hirsutism and obesity, which can complicate care. Disparities in access to costly treatments like GLP-1-analogues may worsen health inequality. There is also concern about healthcare system strain and treatment crowd-out. Given several knowledge gaps, treatment decisions must be individualized, transparent about uncertainties and based on both professional judgment and patient preferences.
Discussion
This report shows that several pharmacological treatments can relieve important symptoms for patients with PCOS, such as irregular menstruation and excess weight. We conclude that metformin could have a small effect on weight loss, however the effect of metformin on metabolic outcomes is modest. GLP-1 analogues may support short-term weight loss but lack solid evidence for broader benefits or safety. As of hirsutism, only CPA-containing pills have some evidence for reducing that outcome, though risks of adverse events limit their use.
COCP will likely improve menstrual regularity in PCOS women in the same manner that is observed in healthy women. It is however not studied what effect a continuous use of COCPs (no pill breaks) would have on the endothelium in women with PCOS.
When assessing the research for all included interventions, we concluded that research is often limited to short follow-up periods, surrogate outcomes, and a narrow age range of study-participants. This means that the long-term health effects for all investigated pharmacological treatments remain unclear.
This is an evidence gap of significance and longer studies are needed in order to investigate if pharmacological treatments can reduce the risk of co-morbidities such as diabetes type 2, cardiovascular disease and endometrial cancer in women with PCOS.
Also, most included studies did not assess quality of life or investigated the effects on mental health. It is important that these outcomes are included in future studies.
Conflict of Interest
The experts and scientific reviewers participating in this project have submitted statements about conflicts of interest. These documents are available at SBU’s secretariat. SBU has determined that the conditions described in the submissions are compatible with SBU’s requirements for objectivity and impartiality.
The full report in Swedish
Läkemedelsbehandling av polycystiskt ovarialsyndrom – hälsa och livskvalitet på kort och lång sikt
Project group
Experts
- Angelica Lindén Hirschberg, Professor, Department of Women’s and Children’s Health, Karolinska Institutet; Senior Physician, Gynecology and Obstetrics, Karolinska University Hospital
- Maria Forslund, Associate Professor, Sahlgrenska Academy, University of Gothenburg; Senior Physician, Gynecology and Obstetrics, Sahlgrenska University Hospital
- Per Wändell, Professor Emeritus in Family Medicine, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet
SBU
- Lisa Forsberg, Project Director
- Marie Österberg, Assistant Project Director
- Jessica Dagerhamn, Assistant Project Director
- Jenny Ågren, Analyst
- Maja Kärrman Fredriksson, Information Specialist
- Emma Wernersson, Project Administrator
- Sigrid Widén, Project Administrator
- Maria Hoppe, Project Administrator
- Anna Ringborg, Health Economist
- Jenny Odeberg, Head of department
Flow chart

 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services
 Share on Facebook
Share on Facebook
 Share on LinkedIn
Share on LinkedIn
 Share via Email
Share via Email