Effectiveness, complications, and health economic and ethical aspects of preimplantation genetic testing for aneuploidy (PGT-A) during in vitro fertilisation (IVF)
HTA Report
Main message
The scientific evidence shows a comparable number of live births after in vitro fertilisation (IVF) with or without preimplantation genetic testing for aneuploidy (PGT-A).
Conclusions
After reviewing the scientific literature, SBU draws the following conclusions:
- The proportion of deliveries with at least one live birth after IVF with PGT-A seems to be comparable to IVF without PGT-A. This is also the case for women over 35 years, although the results are more uncertain for this group.
- Adding PGT-A to IVF almost doubles the cost of an IVF-treatment (defined as an oocyte retrieval and the first embryo transfer) without any increase in live births.
- It is not possible to evaluate if the miscarriage rate is affected when adding PGT-A to IVF since the certainty of evidence is very low.
- There are too few studies to evaluate if PGT-A can reduce the time for IVF treatment to lead to a live born child.
- When comparing biopsy of the embryo with no biopsy in IVF, there does not seem to be any difference in complications for the pregnant women or children.
Aim
The purpose of this systematic review was to evaluate if IVF with PGT-A results in more live births than IVF without PGT-A, and to investigate possible complications from the biopsy. It also includes an analysis of health economic and ethical aspects.
Background
In IVF, embryos are traditionally selected based on morphology to determine which embryo should be transferred to the woman. PGT-A is an additional method for embryo selection that has been suggested to increase the pregnancy and live birth rates. PGT-A involves a biopsy of the embryo (a few cells are taken out) and an analysis of the number of chromosomes. Only embryos with a normal number of chromosomes are transferred to the woman, potentially leading to a higher probability of pregnancy. However, PGT-A also leads to fewer embryos available for transfer and some women will not receive any embryo transfer and it cannot be ruled out that some of the excluded embryos could have resulted in a live birth. The question is therefore whether IVF with PGT-A increases the live birth rate or not. In older women it is more common to have embryos with an abnormal number of chromosomes, suggesting that older women might benefit more from this method. PGT-A is not legally permitted in Sweden but is used in many other countries.
Method
We conducted a systematic review for three separate research questions and reported it in accordance with the PRISMA statement. The protocol is registered in Prospero (CRD42024529876). The certainty of evidence was assessed using GRADE.
Research questions
- What effect does IVF with PGT-A have on outcomes related to pregnancy and quality of life?
- Does embryo biopsy during IVF treatment with preimplantation genetic testing (PGT) result in any complications for the child or the pregnant woman?
- What is the relationship between costs and effects of IVF with PGT-A compared to IVF without PGT-A?
Inclusion criteria
PICO 1: Effectiveness of PGT-A
- Population: Women undergoing IVF treatment .
- Intervention: IVF treatment with PGT-A, where all chromosomes are analysed.
- Control: IVF treatment without PGT-A.
- Outcome: Primary outcomes: Proportion of deliveries with at least one live born child per randomized woman, after the first planned embryo transfer and cumulatively for all embryo transfers from one oocyte retrieval. Secondary outcomes: Proportion of deliveries with at least one live born child per embryo transfer, pregnancy rate, miscarriage rate, ectopic pregnancies, time to a live born child and quality of life for the woman and partner.
- Study design: Randomized controlled trials and systematic reviews following PRISMA.
PICO 2: Complications of PGT
- Population: Children who have been conceived through IVF and women who have undergone an IVF treatment and achieved a pregnancy or delivery.
- Intervention: IVF treatment with biopsy of the embryo to perform any form of preimplantation genetic testing (PGT).
- Control: IVF treatment without biopsy of the embryo.
- Outcome: Perinatal and long-term outcomes for children and placenta-related complications for pregnant women.
- Study design: Controlled studies with or without randomization that compare the outcomes between the intervention and control group. Can be prospective or retrospective studies. Systematic reviews following PRISMA.
PICO 3: Health economic aspects of PGT-A
- Same population, intervention and control as PICO 1.
- Outcomes: Costs, Resource use, Cost per effect
- Study design: Comparative cost analyses and health economic evaluations.
All PICO:s
- Language: Scandinavian or English
- Databases searched: CINAHL (EBSCO), Cochrane Library (Wiley), EMBASE (Embase.com), Medline (Ovid), PsycINFO (EBSCO), PubMed (NLM), Scopus (Elsevier)
In addition to research questions 1-3, we examined ethical issues of relevance for performing IVF with PGT-A in a Swedish context. These issues were identified from qualitative literature found in searches for PICO 1-3, complementary literature searches, discussions in the group working with the report and discussions with patient advocacy groups.
Patient involvement: yes
Result
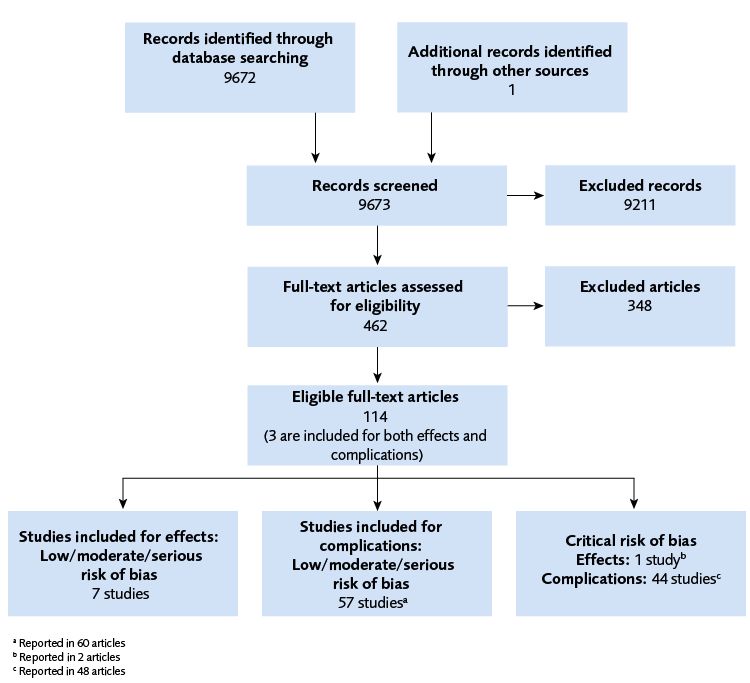
The search for effect and complications resulted in 9 673 references (Figure 1). We included 7 RCTs on the effect of PGT-A and 57 studies on complications from embryo biopsy. Three studies were included for both research questions, see figure 1.
The effect of IVF with PGT-A compared to IVF without PGT-A is presented in table 1. The certainty of evidence (GRADE) was mainly affected by limitations in the included studies that may result in bias, precision due to few studies, or heterogeneity. For complications, a synthesis without meta-analysis was used, and no assessment of the certainty of evidence (GRADE) was done. However, the results do not seem to differ between those women that had IVF with or without biopsy for any of the included complications.
| Abbreviations: ITT = intention to treat analysis. ⊕⊕◯◯ = low certainty of evidence, ⊕◯◯◯= very low certainty of evidence |
||
| Outcome | Results | GRADE |
|---|---|---|
| Deliveries with live birth | ||
| After first planned embryo transfer (ITT) | ||
| All ages ≥35 years |
Comparable effect Cannot be determined |
⊕⊕◯◯ ⊕◯◯◯ |
| All embryo transfers from an oocyte retrieval (ITT) | ||
| All ages ≥35 years |
Comparable effect Comparable effect |
⊕⊕◯◯ ⊕⊕◯◯ |
| For those that had an embryo transfer | ||
| All ages ≥35 years |
In favour of PGT-A In favour of PGT-A |
⊕⊕◯◯ ⊕⊕◯◯ |
| Miscarriage | ||
| Miscarriage per clinical pregnancy | ||
| All ages ≥35 years |
Cannot be determined |
⊕◯◯◯ ⊕◯◯◯ |
Health Economic Assessment
Twelve relevant studies were identified in the literature search, but only two had sufficient quality (at least low to medium quality) and were included in the report. Since these studies were from China and the USA, respectively, the results are expected to have limited transferability to a Swedish context if PGT-A were to be made available. Thus, a health economic model was constructed using data from Swedish registry data for IVF treatment and Swedish costs. Based on the results of the systematic review on the effect of adding PGT-A to IVF, our analyses showed an increase in costs by 37 500 Swedish kronor when adding PGT-A to IVF, without any increase in live births. This result was consistent for the subgroup of women 35 years and older.
Ethics
A healthcare intervention needs to have benefits to patients that outweigh its potential disadvantages. PGT-A does not fulfil this condition as there is no evidence of any relevant patient benefits in our systematic review.
If PGT-A were nevertheless to be offered within publicly funded healthcare in Sweden, priority setting decisions would be needed. Additionally, patients’ informed consent to PGT-A may be threatened by unrealistic expectations. It would therefore be essential that healthcare providers are well informed about the state of scientific knowledge in this area and able to communicate this accurately to patients.
Discussion
In this systematic review we did not find any support for the hypothesis that adding PGT-A to IVF treatment increases the live birth rate when considering all randomised women in the included trials. In addition, no increased live birth rate could be found for women 35 years or older, even though older women have a higher proportion of aneuploid embryos. Our results may be due to fewer women in the group that had IVF with PGT-A having an embryo transfer. It is also possible that some of the embryos excluded after PGT-A analysis could have resulted in a live birth.
For the outcome miscarriage rate no conclusions could be drawn as the result was too uncertain, which may be due to too few studies and events. No synthesis of the result could be performed for the outcome time to live birth as there were too few studies. There is a need for more well-conducted studies focusing on specific groups of patients, to assess whether PGT-A offers benefit to particular populations.
Our results for complications related to the biopsy of the embryo did not show any differences, but the certainty of evidence was not assessed, as the studies were too heterogenous.
In conclusion, PGT-A does not increase the proportion of successful IVF treatments, but adding PGT-A to an IVF treatment nearly doubles the costs. From an ethical perspective, PGT-A thereby does not fulfil the requirement that healthcare interventions should have clear benefits that outweigh their disadvantages.
Conflict of Interest
In accordance with SBU’s requirements, the experts and scientific reviewers participating in this project have submitted statements about conflicts of interest. These documents are available at SBU’s secretariat. SBU has determined that the conditions described in the submissions are compatible with SBU’s requirements for objectivity and impartiality.
The full report in Swedish
Undersökning av avvikande antal kromosomer i embryot vid assisterad befruktning
Project group
Experts
- Christina Bergh, Professor Emerita, Senior Physician, Obstetrics and Gynecology, Sahlgrenska University Hospital and Region Västra Götaland, Gothenburg
- Britt Friberg, Assistant professor, Senior Physician, Obstetrics and Gynecology, Lund IVF Center
- Erik Iwarsson, Assistant Professor, Senior Physician, Clinical Genetics, Karolinska University Hospital, Stockholm
- Kersti Lundin, Assistant Professor, Senior Biologist, University of Gothenburg
- Erik Malmqvist, Assistant Professor in Medical Ethics, Lecturer, University of Gothenburg
SBU
- Susanne Johansson, Project Director
- Fanny Sellberg, Assistant Project Director
- Jan Adolfsson, Assistant Project Director
- Maria Ahlberg, Project Administrator
- Jenny Berg, Health Economist
- Martina Lundqvist, Health Economist
- Maja Kärrman Fredriksson, Information Specialist
- Jenny Ågren, Analyst
- Jenny Odeberg, Head of department
Flow chart
References
- WHO. Infertility Prevalence Estimates, 1990–2021. World Health Organization Team Sexual and Reproductive Health and Research (SRH); 2023. [accessed May 22 2025]. Available from: https://www.who.int/publications/i/item/978920068315.
- Bergh C, Kluge L. Fertilitetsbehandlingar i Sverige. Årsrapport 2024. Gäller behandlingar startade 2022. RESULTAT – TRENDER – ÖPPNA JÄMFÖRELSER. Göteborg: Q-IVF Nationellt kvalitetsregister för assisterad befruktning; 2024. [accessed April 22 2025]. Available from: https://www.medscinet.com/qivf/uploads/hemsida/%C3%85rsrapport%202024%20Final%20version%202.pdf.
- Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. Available from: https://doi.org/10.1016/s0140-6736(78)92957-4.
- Adamson GD, Creighton P, de Mouzon J, Zegers-Hochschild F, Dyer S, Chambers GM. How many infants have been born with the help of assisted reproductive technology? Fertil Steril. 2025. Available from: https://doi.org/10.1016/j.fertnstert.2025.02.009.
- SFOG. Ofrivillig barnlöshet. Svensk förening för obstetrik och gynekologi Arbets- och referensgrupp för ofrivillig barnlöshet; 2010 Report nr 64. [accessed April 22 2025]. Available from: https://www.sfog.se/natupplaga/ARG64d1c6ef01-208e-4626-9b8f-b0da564c61d9.pdf.
- SFOG. Reproduktionsmedicin. Stockholm: Svensk förening för obstetrik och gynekologi Arbets- och referensgrupp för ofrivillig barnlöshet; 2019 Report nr 81. [accessed April 22 2025]. Available from: https://www.sfog.se/natupplaga/ARG81_LR8f508dc3-aec0-45fe-9efc-58a2b2873e50.pdf.
- Handyside AH, Pattinson JK, Penketh RJ, Delhanty JD, Winston RM, Tuddenham EG. Biopsy of human preimplantation embryos and sexing by DNA amplification. Lancet. 1989;1(8634):347-9. Available from: https://doi.org/10.1016/s0140-6736(89)91723-6.
- Volovsky M, Scott RT, Jr., Seli E. Non-invasive preimplantation genetic testing for aneuploidy: is the promise real? Hum Reprod. 2024;39(9):1899-908. Available from: https://doi.org/10.1093/humrep/deae151.
- Barad DH, Albertini DF, Molinari E, Gleicher N. IVF outcomes of embryos with abnormal PGT-A biopsy previously refused transfer: a prospective cohort study. Human Reproduction. 2022;37(6):1194-206. Available from: https://doi.org/10.1093/humrep/deac063.
- Viotti M, Greco E, Grifo JA, Madjunkov M, Librach C, Cetinkaya M, et al. Chromosomal, gestational, and neonatal outcomes of embryos classified as a mosaic by preimplantation genetic testing for aneuploidy. Fertil Steril. 2023;120(5):957-66. Available from: https://doi.org/10.1016/j.fertnstert.2023.07.022.
- Duffy JMN, Bhattacharya S, Bhattacharya S, Bofill M, Collura B, Curtis C, et al. Standardizing definitions and reporting guidelines for the infertility core outcome set: an international consensus development study∗†. Fertility and Sterility. 2021;115(1):201-12. Available from: https://doi.org/10.1016/j.fertnstert.2020.11.013.
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertility and Sterility. 2017;108(3):393-406. Available from: https://doi.org/10.1016/j.fertnstert.2017.06.005.
- Saket Z, Källén K, Lundin K, Magnusson Å, Bergh C. Cumulative live birth rate after IVF: trend over time and the impact of blastocyst culture and vitrification. Human Reproduction Open. 2021;2021(3). Available from: https://doi.org/10.1093/hropen/hoab021.
- Consensus TWGotuotEAI, Coticchio G, Ahlström A, Arroyo G, Balaban B, Campbell A, et al. The Istanbul consensus update: a revised ESHRE/ALPHA consensus on oocyte and embryo static and dynamic morphological assessment†,‡. Human Reproduction. 2025. Available from: https://doi.org/10.1093/humrep/deaf021.
- Glujovsky D, Quinteiro Retamar AM, Alvarez Sedo CR, Ciapponi A, Cornelisse S, Blake D. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2022;5(5):Cd002118. Available from: https://doi.org/10.1002/14651858.CD002118.pub6.
- Kokkali G, Coticchio G, Bronet F, Celebi C, Cimadomo D, Goossens V, et al. ESHRE PGT Consortium and SIG Embryology good practice recommendations for polar body and embryo biopsy for PGT. Hum Reprod Open. 2020;2020(3):hoaa020. Available from: https://doi.org/10.1093/hropen/hoaa020.
- De Vos A, Staessen C, De Rycke M, Verpoest W, Haentjens P, Devroey P, et al. Impact of cleavage-stage embryo biopsy in view of PGD on human blastocyst implantation: a prospective cohort of single embryo transfers. Human Reproduction. 2009;24(12):2988-96. Available from: https://doi.org/10.1093/humrep/dep251.
- Cornelisse S, Zagers M, Kostova E, Fleischer K, van Wely M, Mastenbroek S. Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation. Cochrane Database Syst Rev. 2020;9(9):Cd005291. Available from: https://doi.org/10.1002/14651858.CD005291.pub3.
- Zhang X, Zheng PS. Mechanism of chromosomal mosaicism in preimplantation embryos and its effect on embryo development. J Assist Reprod Genet. 2024;41(5):1127-41. Available from: https://doi.org/10.1007/s10815-024-03048-2.
- Tiegs AW, Tao X, Zhan Y, Whitehead C, Kim J, Hanson B, et al. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing-based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertility and sterility. 2021;115(3):627-37. Available from: https://doi.org/10.1016/j.fertnstert.2020.07.052.
- De Rycke M, Capalbo A, Coonen E, Coticchio G, Fiorentino F, Goossens V, et al. ESHRE survey results and good practice recommendations on managing chromosomal mosaicism. Hum Reprod Open. 2022;2022(4):hoac044. Available from: https://doi.org/10.1093/hropen/hoac044.
- Leigh D, Cram DS, Rechitsky S, Handyside A, Wells D, Munne S, et al. PGDIS position statement on the transfer of mosaic embryos 2021. Reprod Biomed Online. 2022;45(1):19-25. Available from: https://doi.org/10.1016/j.rbmo.2022.03.013.
- SFS 2006:2006:351. Lag (2006:351) om genetisk integritet m.m. Svensk författningssamling. Sveriges riksdag Socialdepartementet. [Available from: https://www.riksdagen.se/sv/dokument-och-lagar/dokument/svensk-forfattningssamling/lag-2006351-om-genetisk-integritet-m.m_sfs-2006-351/.
- Socialstyrelsen 2009:4 kap. 7 § Socialstyrelsens föreskrifter och allmänna råd (SOSFS 2009:32) om användning av vävnader och celler i hälso- och sjukvården och vid klinisk forskning. Socialstyrelsen. [accessed April 22 2025]. Available from: https://www.socialstyrelsen.se/kunskapsstod-och-regler/regler-och-riktlinjer/foreskrifter-och-allmanna-rad/konsoliderade-foreskrifter/200932-om-anvandning-av-vavnader-och-celler-i-halso--och-sjukvarden-och-vid-klinisk-forskning/.
- SKR. Assisterad befruktning Uppföljningsrapport med definitioner, rekommendationer och utvecklingsområden. Stockholm: Sveriges Kommuner och Regioner,; 2014 Version 2. [accessed April 22 2025]. Available from: https://skr.se/download/18.1f376ad3177c89481f73e1af/1615204927279/SKL_assisterad_befruktning_uppfoljningsrapport_052014.pdf.
- SKR. Rekommendation om enhetlighet i regionernas erbjudande av offentlig finansierad assisterad befruktning (revidering 2). Stockholm: Sveriges Kommuner och Regioner; 2020. [accessed April 22 2025]. Available from: https://skr.se/download/18.32563d7d1784aa279ecac6bb/1618304676440/Rekommendation-assisterad%20befruktning-ver2-nov2020.pdf.
- Socialstyrelsen. Preimplantatorisk genetisk diagnostik. Stockholm: Socialstyrelsen,; 2021. [accessed April 22 2025]. Available from: https://www.socialstyrelsen.se/kunskapsstod-och-regler/regler-och-riktlinjer/nationell-hogspecialiserad-vard/oversikt/preimplantatorisk-genetisk-diagnostik/.
- Bergh C, Kluge L. Göteborg: Q-IVF Nationellt kvalitetsregister för assisterad befruktning. [accessed April 22 2025]. Available from: https://www.medscinet.com/qivf/.
- Socialdepartementet Genetisk integritet m.m. Prop. 2005/06:64. Stockholm: Regeringskansliet. [accessed April 22 2025]. Available from: https://www.regeringen.se/rattsliga-dokument/proposition/2006/01/prop.-20050664.
- The European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE), C Calhaz-Jorge, J Smeenk, C Wyns, D De Neubourg, D P Baldani, C Bergh, I Cuevas-Saiz, Ch De Geyter, M S Kupka, K Rezabek, A Tandler-Schneider, V Goossens, Survey on ART and IUI: legislation, regulation, funding, and registries in European countries—an update, Human Reproduction, Volume 39, Issue 9, September 2024, Pages 1909–1924. Available from: https://doi.org/10.1093/humrep/deae163.
- SART National summary report. Preliminary National Summary Report for 2022. [accessed April 2022 2025]. Available from: https://www.sartcorsonline.com/CSR/Public?ClinicPKID=0&reportingYear=2022.
- Ducharme J. IVF Patients Say a Test Caused Them to Discard Embryos. Now They’re Suing. Time. 2025. Available from: https://time.com/7264271/ivf-pgta-test-lawsuit/.
- Tzortzatos G, Pettersson K, Edelstam G, Wånggren K. Cross Border Reproductive Care (CBRC). SFOG; 2019. [accessed March 20 2024]. Available from: https://www.sfog.se/media/336803/cross-border-reproductive-care.pdf.
- Capalbo A, de Wert G, Mertes H, Klausner L, Coonen E, Spinella F, et al. Screening embryos for polygenic disease risk: a review of epidemiological, clinical, and ethical considerations. Human Reproduction Update. 2024;30(5):529-57. Available from: https://doi.org/10.1093/humupd/dmae012.
- Thurin A, Hausken J, Hillensjö T, Jablonowska B, Pinborg A, Strandell A, et al. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med. 2004;351(23):2392-402. Available from: https://doi.org/10.1056/NEJMoa041032.
- SBU. Utvärdering av metoder i hälso- och sjukvården och insatser i socialtjänsten: en metodbok. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2020. [accessed 15 jun 2020]. Available from: https://www.sbu.se/sv/metod/metodboken-2023/.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. Available from: https://doi.org/10.1371/journal.pmed.1000097.
- Bergh C, Lundin K, Iwarsson E, Friberg B, Malmqvist E, Johansson S, et al. Effectiveness and complications of preimplantation genetic testing for aneuploidies (PGT-A) in in vitro fertilisation (IVF). 2024. PROSPERO. Available from: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024529876
- [Effectiveness and complications of preimplantation genetic testing for aneuploidies (PGT-A) in in vitro fertilisation (IVF)]. International HTA Database. [Available from: https://database.inahta.org/article/23916.
- Magee LA, Nicolaides KH, von Dadelszen P. Preeclampsia. N Engl J Med. 2022;386(19):1817-32. Available from: https://doi.org/10.1056/NEJMra2109523.
- The EndNote Team. EndNote. Philadelphia, PA: Clarivate; 2013.
- Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240-3. Available from: https://doi.org/10.3163/1536-5050.104.3.014.
- Covidence. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation. Available from: www.covidence.org.
- Sterne J, Hernán M, McAleenan A, Reeves B, Higgins J. Chapter 25: Assessing risk of bias in a non-randomized study [last updated October 2019]. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Cochrane, 2024. Available from cochrane.org/handbook.
- Frick AP. Advanced maternal age and adverse pregnancy outcomes. Best Pract Res Clin Obstet Gynaecol. 2021;70:92-100. Available from: https://doi.org/10.1016/j.bpobgyn.2020.07.005.
- Giuffrè M, Piro E, Corsello G. Prematurity and twinning. J Matern Fetal Neonatal Med. 2012;25 Suppl 3:6-10. Available from: https://doi.org/10.3109/14767058.2012.712350.
- Koullali B, van Zijl MD, Kazemier BM, Oudijk MA, Mol BWJ, Pajkrt E, et al. The association between parity and spontaneous preterm birth: a population based study. BMC Pregnancy and Childbirth. 2020;20(1):233. Available from: https://doi.org/10.1186/s12884-020-02940-w.
- Lean SC, Derricott H, Jones RL, Heazell AEP. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS One. 2017;12(10):e0186287. Available from: https://doi.org/10.1371/journal.pone.0186287.
- Shah PS. Parity and low birth weight and preterm birth: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010;89(7):862-75. Available from: https://doi.org/10.3109/00016349.2010.486827.
- Siristatidis C, Papapanou M, Karageorgiou V, Martins WP, Bellos I, Teixeira DM, et al. Congenital anomaly and perinatal outcome following blastocyst- vs cleavage-stage embryo transfer: systematic review and network meta-analysis. Ultrasound in Obstetrics & Gynecology. 2023;61(1):12-25. Available from: https://doi.org/10.1002/uog.26019.
- Zaat T, Zagers M, Mol F, Goddijn M, van Wely M, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev. 2021;2(2):Cd011184. Available from: https://doi.org/10.1002/14651858.CD011184.pub3.
- Cimadomo D, Capalbo A, Dovere L, Tacconi L, Soscia D, Giancani A, et al. Leave the past behind: women's reproductive history shows no association with blastocysts' euploidy and limited association with live birth rates after euploid embryo transfers. Hum Reprod. 2021;36(4):929-40. Available from: https://doi.org/10.1093/humrep/deab014.
- Review Manager (RevMan). The Cochrane Collaboration. Available from: https://revman.cochrane.org/info.
- Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.5 (updated August 2024). Cochrane; 2024. [accessed April 23 2025]. Available from: www.training.cochrane.org/handbook.
- Wang L, Wang X, Li M, Liu Y, Ou X, Chen L, et al. PGT-A: The biology and hidden failures of randomized control trials. Prenatal Diagnosis. 2022;42(9):1211-21. Available from: https://doi.org/10.1002/pd.6199.
- Yan J, Qin Y, Zhao H, Sun Y, Gong F, Li R, et al. Live Birth with or without Preimplantation Genetic Testing for Aneuploidy. The New England journal of medicine. 2021;385(22):2047-58. Available from: https://doi.org/10.1056/NEJMoa2103613.
- McKenzie J, Brennan S. Chapter 12: Synthesizing and presenting findings using other methods [last updated October 2019]. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 65: Cochrane; 2024. Available from: https://training.cochrane.org/handbook/current/chapter-12.
- Kato K, Kuroda T, Yamadera-Egawa R, Ezoe K, Aoyama N, Usami A, et al. Preimplantation Genetic Testing for Aneuploidy for Recurrent Pregnancy Loss and Recurrent Implantation Failure in Minimal Ovarian Stimulation Cycle for Women Aged 35–42 Years: Live Birth Rate, Developmental Follow-up of Children, and Embryo Ranking. Reproductive Sciences. 2023;30(3):974-83. Available from: https://doi.org/10.1007/s43032-022-01073-z.
- Kato K, Ezoe K, Onogi S, Ito S, Egawa R, Aoyama N, et al. Comparison of 1-year cumulative live birth and perinatal outcomes following single blastocyst transfer with or without preimplantation genetic testing for aneuploidy: a propensity score-matched study. Journal of assisted reproduction and genetics. 2023;40(11):2669-80. Available from: https://doi.org/10.1007/s10815-023-02926-5.
- Belva F, Kondowe F, De Vos A, Keymolen K, Buysse A, Hes F, et al. Cleavage-stage or blastocyst-stage embryo biopsy has no impact on growth and health in children up to 2 years of age. Reproductive Biology and Endocrinology. 2023;21(1):87. Available from: https://doi.org/10.1186/s12958-023-01140-3.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924. Available from: https://doi.org/10.1136/bmj.39489.470347.AD.
- SBU. Bilaga 8 Mall för kvalitetsgranskning av hälsoekonomiska modellstudier. In: Utvärdering av insatser i hälso- och sjukvården och socialtjänsten: En metodbok. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2023. [accessed April 23 2025]. Available from: https://www.sbu.se/globalassets/ebm/mall_modell_halsoekonomi.pdf.
- Munne S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertility and sterility. 2019;112(6):1071-9.e7. Available from: https://doi.org/10.1016/j.fertnstert.2019.07.1346.
- Ozgur K, Berkkanoglu M, Bulut H, Yoruk GDA, Candurmaz NN, Coetzee K. Single best euploid versus single best unknown-ploidy blastocyst frozen embryo transfers: a randomized controlled trial. Journal of Assisted Reproduction and Genetics. 2019;36(4):629-36. Available from: https://doi.org/10.1007/s10815-018-01399-1.
- Scott Jr RT, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: A randomized controlled trial. Fertility and Sterility. 2013;100(3):697-703. Available from: https://doi.org/10.1016/j.fertnstert.2013.04.035.
- Utomregional prislista samt för EU/EES och Schweiz. Karolinska Universitetssjukhuset,; 2024. [accessed April 23 2025]. Available from: https://www.regionstockholm.se/491c61/contentassets/6f0275ce70be462193c2480734710703/bilaga-2-utomregional-prislista-karolinska-universitetssjukhuset-2024.pdf.
- Besök på barnmorskemottagningen. Region Stockholm: 1177. [accessed April 2023 2025]. Available from: https://www.1177.se/Stockholm/barn--gravid/graviditet/undersokningar-under-graviditeten/besok-pa-barnmorskemottagningen/.
- Socialstyrelsen. Prospektiva viktlistor och statistik om NordDRG. Stockholm: Socialstyrelsen,. [accessed April 23 2025]. Available from: https://www.socialstyrelsen.se/statistik-och-data/klassifikationer-och-koder/drg/viktlistor/.
- Socialstyrelsen. Statistik om graviditeter, förlossningar och nyfödda. Stockholm: Socialstyrelsen,. [accessed April 23 2025]. Available from: https://www.socialstyrelsen.se/statistik-och-data/statistik/alla-statistikamnen/graviditeter-forlossningar-och-nyfodda/.
- SBU. Etiska aspekter på insatser inom hälso- och sjukvården: En vägledning för att identifiera relevanta etiska aspekter. In. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2021. [accessed Feb 29 2024]; p. 1-17. Available from: https://www.sbu.se/globalassets/ebm/etiska_aspekter_halso_sjukvarden.pdf.
- Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. Invitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertility and Sterility. 2013;100(1):100-7.e1. Available from: https://doi.org/10.1016/j.fertnstert.2013.02.056.
- Sui YL, Lei CX, Ye JF, Fu J, Zhang S, Li L, et al. In vitro fertilization with single-Nucleotide polymorphism microarray-based preimplantation genetic testing for aneuploidy significantly improves clinical outcomes in infertile women with recurrent pregnancy loss: A randomized controlled trial. Reproductive and Developmental Medicine. 2020;4(1):32-41. Available from: https://doi.org/10.4103/2096-2924.281852.
- Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Molecular Cytogenetics. 2012;5(1):24. Available from: https://doi.org/10.1186/1755-8166-5-24.
- Yang Z, Salem SA, Liu X, Kuang Y, Salem RD, Liu J. Selection of euploid blastocysts for cryopreservation with array comparative genomic hybridization (aCGH) results in increased implantation rates in subsequent frozen and thawed embryo transfer cycles. Molecular Cytogenetics. 2013;6(1):32. Available from: https://doi.org/10.1186/1755-8166-6-32.
- Rubio C, Bellver J, Rodrigo L, Castillon G, Guillen A, Vidal C, et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertility and sterility. 2017;107(5):1122-9. Available from: https://doi.org/10.1016/j.fertnstert.2017.03.011.
- Verpoest W, Staessen C, Bossuyt PM, Goossens V, Altarescu G, Bonduelle M, et al. Preimplantation genetic testing for aneuploidy by microarray analysis of polar bodies in advanced maternal age: a randomized clinical trial. Human reproduction (Oxford, England). 2018;33(9):1767-76. Available from: https://doi.org/10.1093/humrep/dey262.
- Busnelli A, Somigliana E, Cirillo F, Baggiani A, Levi-Setti PE. Efficacy of therapies and interventions for repeated embryo implantation failure: a systematic review and meta-analysis. Scientific Reports. 2021;11(1):1747. Available from: https://doi.org/10.1038/s41598-021-81439-6.
- Cheng X, Zhang Y, Deng H, Feng Y, Chong W, Hai Y, et al. Preimplantation Genetic Testing for Aneuploidy With Comprehensive Chromosome Screening in Patients Undergoing In Vitro Fertilization: A Systematic Review and Meta-analysis. Obstetrics & Gynecology. 2022;140(5).
- Hou W, Shi G, Ma Y, Liu Y, Lu M, Fan X, et al. Impact of preimplantation genetic testing on obstetric and neonatal outcomes: a systematic review and meta-analysis. Fertility and Sterility. 2021;116(4):990-1000. Available from: https://doi.org/10.1016/j.fertnstert.2021.06.040.
- Kasaven LS, Marcus D, Theodorou E, Jones BP, Saso S, Naja R, et al. Systematic review and meta-analysis: does pre-implantation genetic testing for aneuploidy at the blastocyst stage improve live birth rate? Journal of Assisted Reproduction and Genetics. 2023;40(10):2297-316. Available from: https://doi.org/10.1007/s10815-023-02866-0.
- Liang Z, Wen Q, Li J, Zeng D, Huang P. A systematic review and meta-analysis: clinical outcomes of recurrent pregnancy failure resulting from preimplantation genetic testing for aneuploidy. Frontiers in Endocrinology. 2023;Volume 14 - 2023. Available from: https://doi.org/10.3389/fendo.2023.1178294.
- Mao D, Xu J, Sun L. Impact of trophectoderm biopsy for preimplantation genetic testing on obstetric and neonatal outcomes: a meta-analysis. American Journal of Obstetrics and Gynecology. 2024;230(2):199-212.e5. Available from: https://doi.org/10.1016/j.ajog.2023.08.010.
- Shi W-H, Jiang Z-R, Zhou Z-Y, Ye M-J, Qin N-X, Huang H-F, et al. Different Strategies of Preimplantation Genetic Testing for Aneuploidies in Women of Advanced Maternal Age: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021;10(17):3895.
- Simopoulou M, Sfakianoudis K, Maziotis E, Tsioulou P, Grigoriadis S, Rapani A, et al. PGT-A: who and when? Α systematic review and network meta-analysis of RCTs. Journal of Assisted Reproduction and Genetics. 2021;38(8):1939-57. Available from: https://doi.org/10.1007/s10815-021-02227-9.
- Sordia-Hernandez LH, Morales-Martinez FA, González-Colmenero FD, Flores-Rodriguez A, Leyva-Camacho PC, Sordia-Piñeyro MO, et al. The Effects of Preimplantation Genetic Testing for Aneuploidy (PGT-A) on Patient-Important Outcomes in Embryo Transfer Cases: A Meta-Analysis. J Reprod Infertil. 2022;23(4):231-46. Available from: https://doi.org/10.18502/jri.v23i4.10808.
- Zheng W, Yang C, Yang S, Sun S, Mu M, Rao M, et al. Obstetric and neonatal outcomes of pregnancies resulting from preimplantation genetic testing: a systematic review and meta-analysis. Human Reproduction Update. 2021;27(6):989-1012. Available from: https://doi.org/10.1093/humupd/dmab027.
- Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, et al. In Vitro Fertilization with Preimplantation Genetic Screening. New England Journal of Medicine. 2007;357(1):9-17. Available from: https://doi.org/10.1056/NEJMoa067744.
- Meyer LR, Klipstein S, Hazlett WD, Nasta T, Mangan P, Karande VC. A prospective randomized controlled trial of preimplantation genetic screening in the "good prognosis" patient. Fertility and sterility. 2009;91(5):1731-8. Available from: https://doi.org/10.1016/j.fertnstert.2008.02.162.
- Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M, et al. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19(12):2849-58. Available from: https://doi.org/10.1093/humrep/deh536.
- Staessen C, Verpoest W, Donoso P, Haentjens P, Van der Elst J, Liebaers I, et al. Preimplantation genetic screening does not improve delivery rate in women under the age of 36 following single-embryo transfer. Human Reproduction. 2008;23(12):2818-25. Available from: https://doi.org/10.1093/humrep/den367.
- Desmyttere S, De Schepper J, Nekkebroeck J, De Vos A, De Rycke M, Staessen C, et al. Two-year auxological and medical outcome of singletons born after embryo biopsy applied in preimplantation genetic diagnosis or preimplantation genetic screening. Human Reproduction. 2009;24(2):470-6. Available from: https://doi.org/10.1093/humrep/den402.
- Desmyttere S, De Rycke M, Staessen C, Liebaers I, De Schrijver F, Verpoest W, et al. Neonatal follow-up of 995 consecutively born children after embryo biopsy for PGD. Hum Reprod. 2012;27(1):288-93. Available from: https://doi.org/10.1093/humrep/der360.
- Winter C, Van Acker F, Bonduelle M, Desmyttere S, De Schrijver F, Nekkebroeck J. Cognitive and psychomotor development of 5- to 6-year-old singletons born after PGD: a prospective case–controlled matched study. Human Reproduction. 2014;29(9):1968-77. Available from: https://doi.org/10.1093/humrep/deu165.
- Winter C, Van Acker F, Bonduelle M, Desmyttere S, Nekkebroeck J. Psychosocial development of full term singletons, born after preimplantation genetic diagnosis (PGD) at preschool age and family functioning: a prospective case-controlled study and multi-informant approach. Human Reproduction. 2015;30(5):1122-36. Available from: https://doi.org/10.1093/humrep/dev036.
- Gulersen M, Peyser A, Ferraro A, Goldman R, Mullin C, Li X, et al. Maternal and neonatal outcomes in pregnancies conceived after preimplantation genetic testing. Prenatal Diagnosis. 2021;41(7):835-42. Available from: https://doi.org/10.1002/pd.5937.
- Hao Y, Long X, Kong F, Chen L, Chi H, Zhu X, et al. Maternal and neonatal outcomes following blastocyst biopsy for PGT in single vitrified–warmed embryo transfer cycles. Reproductive BioMedicine Online. 2022;44(1):151-62. Available from: https://doi.org/10.1016/j.rbmo.2021.07.016.
- Ji H, Zhang MQ, Zhou Q, Zhang S, Dong L, Li XL, et al. Trophectoderm biopsy is associated with adverse obstetric outcomes rather than neonatal outcomes. BMC Pregnancy Childbirth. 2023;23(1):141. Available from: https://doi.org/10.1186/s12884-023-05466-z.
- Lewis S, Amor DJ, Glynn A, Wilton L, Halliday J. Child health after preimplantation genetic testing. Reproductive BioMedicine Online. 2021;42(3):609-19. Available from: https://doi.org/10.1016/j.rbmo.2020.11.014.
- Li M, Kort J, Baker VL. Embryo biopsy and perinatal outcomes of singleton pregnancies: an analysis of 16,246 frozen embryo transfer cycles reported in the Society for Assisted Reproductive Technology Clinical Outcomes Reporting System. American Journal of Obstetrics & Gynecology. 2021;224(5):500.e1-.e18. Available from: https://doi.org/10.1016/j.ajog.2020.10.043.
- Li S, Ma S, Zhao J, Hu J, Li H, Zhu Y, et al. Non-Assisted Hatching Trophectoderm Biopsy Does Not Increase The Risks of Most Adverse Maternal and Neonatal Outcome and May Be More Practical for Busy Clinics: Evidence From China. Front Endocrinol (Lausanne). 2022;13:819963. Available from: https://doi.org/10.3389/fendo.2022.819963.
- Li Y, Wen Q, Liao J, Ma S, Zhang S, Gu Y, et al. Trophectoderm Biopsy Differentially Influences the Level of Serum β-Human Chorionic Gonadotropin With Different Embryonic Trophectoderm Scores in Early Pregnancy From 7847 Single-Blastocyst Transfer Cycles. Frontiers in Endocrinology. 2022;13. Available from: https://doi.org/10.3389/fendo.2022.794720.
- Lu M-m, Wen Y-x, Liu Y-l, Ding C-h, Zhou C-q, Xu Y-w. Trophectoderm biopsy reduces the level of serum beta-human chorionic gonadotropin in early pregnancy. Fertility and Sterility. 2020;114(4):801-8. Available from: https://doi.org/10.1016/j.fertnstert.2020.05.015.
- Makhijani R, Bartels CB, Godiwala P, Bartolucci A, DiLuigi A, Nulsen J, et al. Impact of trophectoderm biopsy on obstetric and perinatal outcomes following frozen–thawed embryo transfer cycles. Human Reproduction. 2021;36(2):340-8. Available from: https://doi.org/10.1093/humrep/deaa316.
- Nekkebroeck J, Bonduelle M, Desmyttere S, Van den Broeck W, Ponjaert-Kristoffersen I. Mental and psychomotor development of 2-year-old children born after preimplantation genetic diagnosis/screening. Human Reproduction. 2008;23(7):1560-6. Available from: https://doi.org/10.1093/humrep/den033.
- Nekkebroeck J, Bonduelle M, Desmyttere S, Van den Broeck W, Ponjaert-Kristoffersen I. Socio-emotional and language development of 2-year-old children born after PGD/PGS, and parental well-being. Human Reproduction. 2008;23(8):1849-57. Available from: https://doi.org/10.1093/humrep/den179.
- Ricciarelli E, Bruna I, Verdú V, Torrelló MJ, Herrer R, Gris JM, et al. Impact of assisted reproduction treatments on Spanish newborns: report of 14,119 pregnancies. Journal of Assisted Reproduction and Genetics. 2013;30(7):897-905. Available from: https://doi.org/10.1007/s10815-013-0023-0.
- Roeca C, Johnson R, Carlson N, Polotsky AJ. Preimplantation genetic testing and chances of a healthy live birth amongst recipients of fresh donor oocytes in the United States. Journal of Assisted Reproduction and Genetics. 2020;37(9):2283-92. Available from: https://doi.org/10.1007/s10815-020-01874-8.
- Sun N, Fang X, Jiao Y, Wang Y, Wan Y, Wu Z, et al. Adverse maternal and neonatal outcomes of preimplantation genetic testing with trophectoderm biopsy: a retrospective cohort study of 3373 intracytoplasmic sperm injection single frozen-thawed blastocyst transfer cycles. Arch Gynecol Obstet. 2024;309(6):2427-37. Available from: https://doi.org/10.1007/s00404-023-07120-7.
- Sunkara SK, Antonisamy B, Selliah HY, Kamath MS. Pre-term birth and low birth weight following preimplantation genetic diagnosis: analysis of 88 010 singleton live births following PGD and IVF cycles. Human Reproduction. 2017;32(2):432-8. Available from: https://doi.org/10.1093/humrep/dew317.
- Verpoest W, Van Landuyt L, Desmyttere S, Cremers A, Devroey P, Liebaers I. The incidence of monozygotic twinning following PGD is not increased. Human Reproduction. 2009;24(11):2945-50. Available from: https://doi.org/10.1093/humrep/dep280.
- Wu Y, Ying Y, Cao M, Liu J, Liu H. Trophectoderm biopsy of blastocysts for a preimplantation genetic test does not affect serum β-hCG levels in early pregnancy: a study using propensity score matching. Journal of Ovarian Research. 2021;14(1):78. Available from: https://doi.org/10.1186/s13048-021-00824-x.
- Zheng W, Ren B, Mu M, Liu Y, Liu X, Yang C, et al. Perinatal Outcomes of Singleton Live Births Following Preimplantation Genetic Testing for Chromosomal Structural Rearrangements in Single Frozen-Thawed Blastocyst Transfer Cycles: a Retrospective Cohort Study. Reprod Sci. 2022;29(10):3039-46. Available from: https://doi.org/10.1007/s43032-021-00732-x.
- Cozzolino M, Cecchino GN, Garcia Velasco JA, Pellicer N, Galliano D, Pellicer A. Preimplantation genetic testing for aneuploidy is not related to adverse obstetric and neonatal outcomes in singleton pregnancies. Human Reproduction. 2023;38(8):1621-7. Available from: https://doi.org/10.1093/humrep/dead123.
- Forman EJ, Tao X, Ferry KM, Taylor D, Treff NR, Scott RT, Jr. Single embryo transfer with comprehensive chromosome screening results in improved ongoing pregnancy rates and decreased miscarriage rates. Human Reproduction. 2012;27(4):1217-22. Available from: https://doi.org/10.1093/humrep/des020.
- Gulersen M, Peyser A, Kim J, Ferraro A, Goldman R, Mullin C, et al. The impact of preimplantation genetic testing for aneuploidy on prenatal screening. 2022;50(3):300-4. Available from: https://doi.org/10.1515/jpm-2021-0495.
- Liu D, Chen C, Huang Q, Dong Y, Xu L, Dong M, et al. Preimplantation genetic testing for complex chromosomal rearrangements: clinical outcomes and potential risk factors. Frontiers in Genetics. 2024;15. Available from: https://doi.org/10.3389/fgene.2024.1401549.
- Mejia RB, Capper EA, Summers KM, Mancuso AC, Sparks AE, Van Voorhis BJ. Cumulative live birth rate in women aged ≤37 years after in vitro fertilization with or without preimplantation genetic testing for aneuploidy: a Society for Assisted Reproductive Technology Clinic Outcome Reporting System retrospective analysis. F&S Reports. 2022;3(3):184-91. Available from: https://doi.org/10.1016/j.xfre.2022.05.004.
- Richardson H, Kalliora C, Mainigi M, Coutifaris C, Sammel MD, Senapati S. Impact of mode of conception on early pregnancy human chorionic gonadotropin rise and birth weight. F&S reports. 2022;3(1):13-9. Available from: https://doi.org/10.1016/j.xfre.2021.12.006.
- Riestenberg CK, Mok T, Ong JR, Platt LD, Han CS, Quinn MM. Sonographic abnormalities in pregnancies conceived following IVF with and without preimplantation genetic testing for aneuploidy (PGT-A). Journal of Assisted Reproduction and Genetics. 2021;38(4):865-71. Available from: https://doi.org/10.1007/s10815-021-02069-5.
- Sarkar P, New EP, Jindal S, Tanner JP, Imudia AN. The effect of trophectoderm biopsy for preimplantation genetic testing on fetal birth weight and preterm delivery. Minerva Obstet Gynecol. 2023. Available from: https://doi.org/10.23736/s2724-606x.22.05196-x.
- Shi X, Tang Y, Liu C, Li W, Lin H, Mao W, et al. Effects of NGS-based PGT-a for idiopathic recurrent pregnancy loss and implantation failure: a retrospective cohort study. Systems Biology in Reproductive Medicine. 2023;69(5):354-65. Available from: https://doi.org/10.1080/19396368.2023.2225679.
- Belva F, Roelants M, Kluijfhout S, Winter C, De Schrijver F, Desmyttere S, et al. Body composition and blood pressure in 6-year-old singletons born after pre-implantation genetic testing for monogenic and structural chromosomal aberrations: a matched cohort study. Human Reproduction Open. 2018;2018(4). Available from: https://doi.org/10.1093/hropen/hoy013.
- El-Toukhy T, Kamal A, Wharf E, Grace J, Bolton V, Khalaf Y, et al. Reduction of the multiple pregnancy rate in a preimplantation genetic diagnosis programme after introduction of single blastocyst transfer and cryopreservation of blastocysts biopsied on Day 3. Human Reproduction. 2009;24(10):2642-8. Available from: https://doi.org/10.1093/humrep/dep172.
- Feldman B, Orvieto R, Weisel M, Aizer A, Meyer R, Haas J, et al. Obstetric and Perinatal Outcomes in Pregnancies Conceived After Preimplantation Genetic Testing for Monogenetic Diseases. Obstetrics & Gynecology. 2020;136(4):782-91. Available from: https://doi.org/10.1097/aog.0000000000004062.
- Ginstrom Ernstad E, Hanson C, Wanggren K, Thurin-Kjellberg A, Hulthe Soderberg C, Syk Lundberg E, et al. Preimplantation genetic testing and child health: a national register-based study. Human reproduction (Oxford, England). 2023;38(4):739-50. Available from: https://doi.org/10.1093/humrep/dead021.
- Hasson J, Limoni D, Malcov M, Frumkin T, Amir H, Shavit T, et al. Obstetric and neonatal outcomes of pregnancies conceived after preimplantation genetic diagnosis: cohort study and meta-analysis. Reproductive BioMedicine Online. 2017;35(2):208-18. Available from: https://doi.org/10.1016/j.rbmo.2017.05.003.
- Snelgrove JW, Lee R, Jeyakumar Y, Greenblatt EM, Kingdom JC, Zwingerman R, et al. Maternal Placental Growth Factor (PlGF) levels, sonographic placental parameters, and outcomes of IVF pregnancies with and without embryo trophectoderm biopsy. Journal of assisted reproduction and genetics. 2024;41(10):2721-6. Available from: https://doi.org/10.1007/s10815-024-03193-8.
- Awadalla MS, Park KE, Latack KR, McGinnis LK, Ahmady A, Paulson RJ. Influence of Trophectoderm Biopsy Prior to Frozen Blastocyst Transfer on Obstetrical Outcomes. Reproductive Sciences. 2021;28(12):3459-65. Available from: https://doi.org/10.1007/s43032-021-00552-z.
- Eldar-Geva T, Srebnik N, Altarescu G, Varshaver I, Brooks B, Levy-Lahad E, et al. Neonatal outcome after preimplantation genetic diagnosis. Fertility and sterility. 2014;102(4):1016-21. Available from: https://doi.org/10.1016/j.fertnstert.2014.06.023.
- He H, Jing S, Lu CF, Tan YQ, Luo KL, Zhang SP, et al. Neonatal outcomes of live births after blastocyst biopsy in preimplantation genetic testing cycles: a follow-up of 1,721 children. Fertility and sterility. 2019;112(1):82-8. Available from: https://doi.org/10.1016/j.fertnstert.2019.03.006.
- Kamath M, Antonisamy B, Sunkara S. Zygotic splitting following embryo biopsy: a cohort study of 207 697 single-embryo transfers following IVF treatment. BJOG: An International Journal of Obstetrics & Gynaecology. 2020;127(5):562-9. Available from: https://doi.org/10.1111/1471-0528.16045.
- Liu AH-C, Shah T, Wu H, Lieman HJ, Singh M, Pollack SE, et al. Trophectoderm biopsy is associated with lower risks of moderate to extreme prematurity and low birthweights: a national registry cohort study of singleton livebirths from frozen-thawed blastocyst transfers. American journal of obstetrics and gynecology. 2024. Available from: https://doi.org/10.1016/j.ajog.2024.07.007.
- Sites CK, Bachilova S, Gopal D, Cabral HJ, Coddington CC, Stern JE. Embryo biopsy and maternal and neonatal outcomes following cryopreserved-thawed single embryo transfer. American Journal of Obstetrics and Gynecology. 2021;225(3):285.e1-.e7. Available from: https://doi.org/10.1016/j.ajog.2021.04.235.
- Sun Q, Xu J, Yao Y, Huang X, Zhao D, Lu S, et al. Efficacy of non-invasive chromosome screening, preimplantation genetic testing for aneuploidy, and morphological grading in selecting embryos of patients with advanced maternal age: a three-armed prospective cohort study. BMC pregnancy and childbirth. 2024;24(1):545. Available from: https://doi.org/10.1186/s12884-024-06736-0.
- Zhang WY, von Versen-Höynck F, Kapphahn KI, Fleischmann RR, Zhao Q, Baker VL. Maternal and neonatal outcomes associated with trophectoderm biopsy. Fertility and Sterility. 2019;112(2):283-90.e2. Available from: https://doi.org/10.1016/j.fertnstert.2019.03.033.
- Srebnik N, Sverdlik Kislasi Y, Amosi-Victor D, Rotshenker-Olshinka K, Eldar-Geva T, Ben-Ami I, et al. PGT pregnancies have a similar risk for post-partum complications as naturally conceived pregnancies. Reproductive BioMedicine Online. 2023;46(1):189-95. Available from: https://doi.org/10.1016/j.rbmo.2022.09.009.
- Zheng W, Yang SH, Yang C, Ren BN, Sun SM, Liu YL, et al. Perinatal outcomes of singleton live births after preimplantation genetic testing during single frozen-thawed blastocyst transfer cycles: a propensity score-matched study. Fertility and sterility. 2022;117(3):562-70. Available from: https://doi.org/10.1016/j.fertnstert.2021.12.020.
- SBU. 11. Ekonomiska aspekter. In: Utvärdering av metoder i hälso- och sjukvården och insatser i socialtjänsten: en metodbok. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2020. [accessed April 24 2025]. Available from: https://www.sbu.se/sv/metod/metodboken-2023/.
- He X, Wang X, Shen J, Wan B, Wang Y, Zhang Z, et al. Cost-effectiveness of preimplantation genetic testing for aneuploidy for women with subfertility in China: an economic evaluation using evidence from the CESE-PGS trial. BMC pregnancy and childbirth. 2023;23(1):254. Available from: https://doi.org/10.1186/s12884-023-05563-z.
- Collins SC, Xu X, Mak W. Cost-effectiveness of preimplantation genetic screening for women older than 37 undergoing in vitro fertilization. Journal of Assisted Reproduction and Genetics. 2017;34(11):1515-22. Available from: https://doi.org/10.1007/s10815-017-1001-8.
- Chang J, Boulet SL, Jeng G, Flowers L, Kissin DM. Outcomes of in vitro fertilization with preimplantation genetic diagnosis: an analysis of the United States Assisted Reproductive Technology Surveillance Data, 2011–2012. Fertility and Sterility. 2016;105(2):394-400. Available from: https://doi.org/10.1016/j.fertnstert.2015.10.018.
- Socialdepartementet Proposition 1996/97:60 Prioriteringar inom hälso- och sjukvården. Stockholm: Sveriges riksdag. [accessed April 25 2025]. Available from: https://www.riksdagen.se/sv/dokument-och-lagar/dokument/proposition/prioriteringar-inom-halso-och-sjukvarden_gk0360/.
- WHO. Infertility. [accessed 2025 May 22]. Available from: https://www.who.int/news-room/fact-sheets/detail/infertility.
- Elenkov A, Giwercman A, Søgaard Tøttenborg S, Bonde JPE, Glazer CH, Haervig KK, et al. Male childlessness as independent predictor of risk of cardiovascular and all-cause mortality: A population-based cohort study with more than 30 years follow-up. PLOS ONE. 2020;15(9):e0237422. Available from: https://doi.org/10.1371/journal.pone.0237422.
- Zhang J, Rubin LR, Zierhut H, Pastore LM. Comparison of Patients’ Ethical Perspectives of Preimplantation Embryo Genetic Testing for Aneuploidy (PGT-A) vs. Monogenic Disorders (PGT-M). Reproductive Sciences. 2021;28(11):3272-81. Available from: https://doi.org/10.1007/s43032-021-00644-w.
- Bracewell-Milnes T, Saso S, Jones B, Cato S, Parikh R, Thum MY, et al. A systematic review exploring the patient decision-making factors and attitudes towards pre-implantation genetic testing for aneuploidy and gender selection. Acta Obstet Gynecol Scand. 2021;100(1):17-29. Available from: https://doi.org/10.1111/aogs.13973.
- Kaing A, Rosen MP, Quinn MM. Perceptions, motivations and decision regret surrounding preimplantation genetic testing for aneuploidy. Hum Reprod. 2020;35(9):2047-57. Available from: https://doi.org/10.1093/humrep/deaa154.
- Quinn MM, Juarez-Hernandez F, Dunn M, Okamura RJ, Cedars MI, Rosen MP. Decision-making surrounding the use of preimplantation genetic testing for aneuploidy reveals misunderstanding regarding its benefit. J Assist Reprod Genet. 2018;35(12):2155-9. Available from: https://doi.org/10.1007/s10815-018-1337-8.
- Galiano V, Orvieto R, Machtinger R, Nahum R, Garzia E, Sulpizio P, et al. “Add-Ons” for Assisted Reproductive Technology: Do Patients Get Honest Information from Fertility Clinics’ Websites? Reproductive Sciences. 2021;28(12):3466-72. Available from: https://doi.org/10.1007/s43032-021-00601-7.
- Cheng L, Meiser B, Kennedy D, Kirk E, Barlow-Stewart K, Kaur R. Exploration of decision-making regarding the transfer of mosaic embryos following preimplantation genetic testing: a qualitative study. Hum Reprod Open. 2022;2022(4):hoac035. Available from: https://doi.org/10.1093/hropen/hoac035.
- The Disability Rights Critique of Prenatal Genetic Testing: Reflections and Recommendations. Hastings Center Report. 1999;29(5):s1-s24. Available from: https://doi.org/10.2307/3527746.
- Bayefsky MJ, Shaw J, Hamer D, Martel R, Reich J, Blakemore JK. A balancing act: sex selection after pre-implantation genetic testing for aneuploidy for first versus second baby. Human Reproduction. 2023;38(7):1325-31. Available from: https://doi.org/10.1093/humrep/dead101.
- Dondorp W, De Wert G, Pennings G, Shenfield F, Devroey P, Tarlatzis B, et al. ESHRE Task Force on ethics and Law 20: sex selection for non-medical reasons†. Human Reproduction. 2013;28(6):1448-54. Available from: https://doi.org/10.1093/humrep/det109.
- Barlevy D, Cenolli I, Campbell T, Furrer R, Mukherjee M, Kostick-Quenet K, et al. Patient interest in and clinician reservations on polygenic embryo screening: a qualitative study of stakeholder perspectives. Journal of Assisted Reproduction and Genetics. 2024;41(5):1221-31. Available from: https://doi.org/10.1007/s10815-024-03074-0.
- Furrer RA, Barlevy D, Pereira S, Carmi S, Lencz T, Lázaro-Muñoz G. Public Attitudes, Interests, and Concerns Regarding Polygenic Embryo Screening. JAMA Network Open. 2024;7(5):e2410832-e. Available from: https://doi.org/10.1001/jamanetworkopen.2024.10832.
- Lencz T, Sabatello M, Docherty A, Peterson RE, Soda T, Austin J, et al. Concerns about the use of polygenic embryo screening for psychiatric and cognitive traits. The Lancet Psychiatry. 2022;9(10):838-44. Available from: https://doi.org/10.1016/S2215-0366(22)00157-2.
- SMER. Smer föreslår: Tillsätt en utredning som tar fram en strategi för genteknikområdet och ser över lagstiftningen som skyddar människan. Statens medinsk-etiska råd; 2018. [accessed May 22 2025]. Available from: https://smer.se/wp-content/uploads/2018/06/Skrivelse-om-utredning-av-lagstiftning-f%C3%B6r-ny-genteknik.pdf.
- De Neubourg D, Bogaerts K, Blockeel C, Coetsier T, Delvigne A, Devreker F, et al. How do cumulative live birth rates and cumulative multiple live birth rates over complete courses of assisted reproductive technology treatment per woman compare among registries? Hum Reprod. 2016;31(1):93-9. Available from: https://doi.org/10.1093/humrep/dev270.
- Babariya D, Gill P, Ottolini CS, Mulas F, Picchetta L, Caroselli S, et al. O-155 Limited utility of mosaicism reporting in PGT-A: independent confirmation from a European multisite, double-blinded analysis including 4293 embryo transfers following the first US experience. Human Reproduction. 2024;39(Supplement_1). Available from: https://doi.org/10.1093/humrep/deae108.174.
- Spinella F, Greco E, Madjunkova S, Besser A, Biricik A, Listorti I, et al. O-154 Chromosomal, gestational, and neonatal outcomes of mosaic embryos: analysis of 3074 cases from the international registry of mosaic embryo. Human Reproduction. 2024;39(Supplement_1). Available from: https://doi.org/10.1093/humrep/deae108.173.
- Scott RT, Jr., Upham KM, Forman EJ, Zhao T, Treff NR. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: a randomized and paired clinical trial. Fertil Steril. 2013;100(3):624-30. Available from: https://doi.org/10.1016/j.fertnstert.2013.04.039.
- Lundin K, Bentzen JG, Bozdag G, Ebner T, Harper J, Le Clef N, et al. Good practice recommendations on add-ons in reproductive medicine†. Hum Reprod. 2023;38(11):2062-104. Available from: https://doi.org/10.1093/humrep/dead184.
- ASRM. Washington, DC: American Society for Reproductive Medicine. [accessed April 25 2025]. Available from: https://www.asrm.org/practice-guidance/practice-committee-documents/the-use-of-preimplantation-genetic-testing-for-aneuploidy-a-committee-opinion-2024/.
- HFEA. Human Fertilisation and Embryology Authority. [accessed April 25 2025]. Available from: https://www.hfea.gov.uk/treatments/treatment-add-ons/pre-implantation-genetic-testing-for-aneuploidy-pgt-a/.
- Essers R, Lebedev IN, Kurg A, Fonova EA, Stevens SJC, Koeck RM, et al. Prevalence of chromosomal alterations in first-trimester spontaneous pregnancy loss. Nature Medicine. 2023;29(12):3233-42. Available from: https://doi.org/10.1038/s41591-023-02645-5.
- Berntsen S, Laivuori H, la Cour Freiesleben N, Loft A, Söderström-Anttila V, B Oldereid N, et al. A systematic review and meta-analysis on the association between ICSI and chromosome abnormalities. Human Reproduction Update. 2021;27(5):801-47. Available from: https://doi.org/10.1093/humupd/dmab005.
- Fenwick E, Eze A, D'Hooghe T, Pandey S, Chaudhari VS, Ostawal A, et al. The value of treatment for infertility: A systematic literature review of willingness-to-pay thresholds and approaches for determining the cost effectiveness of fertility therapies. Best Pract Res Clin Obstet Gynaecol. 2023;89:102340. Available from: https://doi.org/10.1016/j.bpobgyn.2023.102340.
- Keller E, Chambers GM. Valuing infertility treatment: Why QALYs are inadequate, and an alternative approach to cost-effectiveness thresholds. Front Med Technol. 2022;4:1053719. Available from: https://doi.org/10.3389/fmedt.2022.1053719.
- Rubio C, Navarro-Sánchez L, García-Pascual CM, Ocali O, Cimadomo D, Venier W, et al. Multicenter prospective study of concordance between embryonic cell-free DNA and trophectoderm biopsies from 1301 human blastocysts. Am J Obstet Gynecol. 2020;223(5):751.e1-.e13. Available from: https://doi.org/10.1016/j.ajog.2020.04.035.

 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services
 Share on Facebook
Share on Facebook
 Share on LinkedIn
Share on LinkedIn
 Share via Email
Share via Email