Diagnostics of ovarian cancer; test accuracy of algorithm-based diagnostics in cases of suspected ovarian cancer.
A systematic review including ethical aspects and overview of health economic considerations.
Main message
SBU assessed six algorithms (SR, PR, RMI, ROMA, ADNEX, LR21) for the evaluation of pre-operative ovarian cancer risk. The most frequently used algorithms in Sweden are PR and SR. General finding: SR and PR demonstrated comparable diagnostic accuracy to most alternatives for the whole population.
1. SR: Simple Rules, PR: Pattern Recognition, RMI: Risk of Malignancy Index, ROMA: Risk of Ovarian Malignancy Algorithm, ADNEX: Assessment of Different NEoplasias in the adneXa, LR2: Logistic Regression Model 2.
Conclusions
- For premenopausal women, using indirect comparisons: SR, PR, and ADNEX algorithms exhibited superior test accuracy.
- For postmenopausal women, SR, PR, RMI, ROMA, and ADNEX algorithms showed high test accuracy, which was indistinguishable from each other.
- For LR2, there was insufficient data to make firm conclusions.
Aim
The aim of this systematic review was to assess the diagnostic test accuracy of six algorithms used for suspected ovarian cancer. It encompasses an ethical discourse on the findings.
Background
The vague symptoms of ovarian cancer complicate early diagnosis for patients and physicians, though timely detection strongly correlates with improved survival. Definitive diagnosis requires microscopic tissue analysis or structured clinical follow-up. Preoperative biopsy is contraindicated due to cancer dissemination risk. Diagnostic algorithms for preoperative evaluation integrate menopausal status, ultrasound findings, and serum tumor markers.
In Sweden, ultrasound – based methods Simple Rules and Pattern Recognition – are predominantly utilised.
Method
We performed a systematic review following the PRISMA guidelines. The certainty of the evidence was evaluated using GRADE.
PIRO
Population: Pre- and postmenopausal (reported separately) women with suspected ovarian, fallopian tube or peritoneal tumors or symptoms indicative of ovarian cancer.
Indextest: The algorithms: RMI (Risk of Malignancy Index), ROMA (Risk of Ovarian Malignancy Algorithm), ADNEX (Assessment of Different NEoplasias in the adneXa model), LR2 (Logistic Regression 2), SR (Simple Rules), and PR (Pattern Recognition).
Reference test: Surgery: a morphological microscopic diagnosis (by a pathologist) or structured follow-up with no development of a diagnosis requiring surgery for at least 12 months, the variants of were considered equivalent.
Outcome: Principle outcome was sensitivity, specificity, and other derivatives from the 2x2 tables (number of true/false positive and true/false negative results)
Study design: Diagnostic prospective or retrospective cross-sectional studies and randomized studies.
Language: English, Swedish, Norwegian, and Danish.
Databases searched: Cochrane Library, CDSR, Central (Wiley), Embase (Elsevier), Medline (Ovid), Clinicaltrials.gov (NLM), WHO ICTRP (WHO).
Result
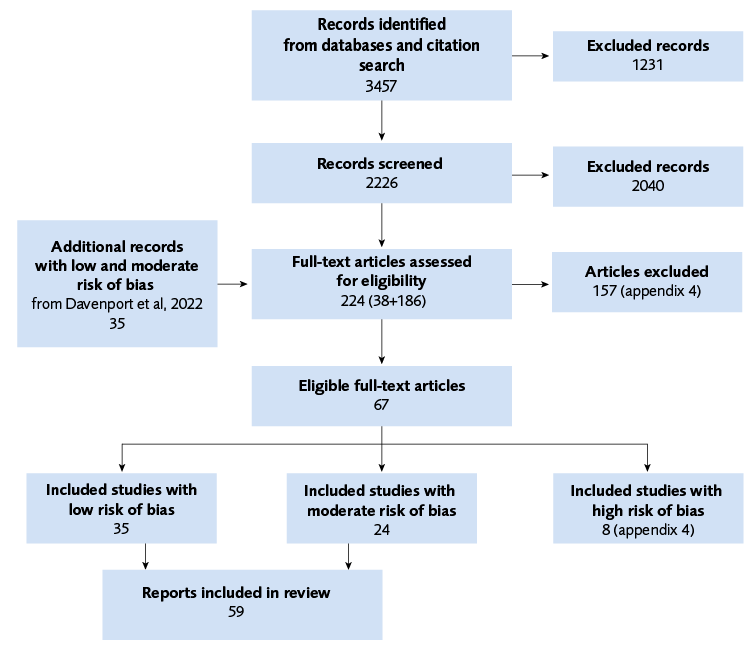
A total of 59 primary studies were included in the analysis, with a total of close to 71500 observations.
| Algorithm (threshold) |
Total number of observations Number of included studies |
Sensitivity (95 % CI) GRADE |
Specificity (95 % CI) GRADE |
Comments on GRADE evaluation |
|---|---|---|---|---|
| RMI (200 IU/ml) |
8229 18 |
0.57 (0.48–0.66) ⊕⊕◯◯ |
0.94 (0.92–0.96) ⊕⊕⊕⊕ |
Sensitivity:-1 heterogeneity, -1 precision Specitivity: / |
| ROMA (11,4–12,5%) |
6150 26 |
0.74 (0.68–0.79) ⊕⊕◯◯ |
0.85 (0.82–0.87) ⊕⊕⊕⊕ |
Sensitivity: -2 precision Specitivity: / |
| LR2 (10%) |
4578 4 |
0.82 (0.82–0.83) ⊕⊕⊕◯ |
0.89 (0.89–0.90) ⊕⊕⊕◯ |
Sensitivity and Specitivity: -1 combination of heterogeneity and limited obser-vations respectively |
| ADNEX (10% and with CA125) |
6843 13 |
0.91 (0.88–0.93) ⊕⊕⊕◯ |
0.86 (0.81–0.89) ⊕⊕⊕◯ |
Sensitivity and Specitivity: -1 heterogeneity respectively |
| SR (according to IOTA guidelines, varying handling of in-conclusive results) |
5453 9 |
0.89 (0.83–0.93) ⊕⊕⊕◯ |
0.95 (0.88–0.98) ⊕⊕⊕◯ |
Sensitivity and Specitivity: -1 combination of heterogeneity and precision |
| PR (ultrasound performed by an experienced sonographer) |
5280 7 |
0.92 (0.85–0.96) ⊕⊕⊕◯ |
0.93 (0.90–0.95) ⊕⊕⊕⊕ |
Sensitivity: -1 combination of heterogeneity and precision Specitivity: / |
There was no significant difference in diagnostic accuracy among ADNEX, PR, and SR. These methods demonstrated superior accuracy relative to RMI and ROMA. The distinction between RMI and ROMA was significant. RMI exhibited lower sensitivity but greater specificity. Conversely, ROMA showed higher sensitivity with reduced specificity. The point estimate for LR2 aligned more closely with ADNEX, PR, and SR, nonetheless, uncertainty existed for LR2 because of insufficient observations to calculate a confidence region.
| Algorithm (threshold) |
Total number of observations Number of included studies |
Sensitivity (95 % CI) GRADE |
Specificity (95 % CI) GRADE |
Comments on GRADE evaluation |
|---|---|---|---|---|
| RMI (200 IU/ml) |
8731 22 |
0.87 (0,83–0,91) ⊕⊕⊕◯ |
0.79 (0,73–0,84) ⊕⊕◯◯ |
Sensibility: -1 heterogeneity Specificity: -1 heterogeneity, -1 precision |
| ROMA (14,4–29,9%) |
7896 32 |
0.87 (0,82–0,91) ⊕⊕◯◯ |
0.83 (0,80–0,86) ⊕⊕⊕◯ |
Sensibility: -1 heterogeneity, -1 precision Specificity: -1 heterogeneity |
| LR2 (10%) |
3735 5 |
0.91 (0.91–0.92) ⊕⊕⊕◯ |
0.66 (0.66–0.77) ⊕⊕◯◯ |
Sensibility: -1 combination of heterogeneity and limited observations Specificity: -1 heterogeneity and limited observations |
| ADNEX (10% and with CA125) |
6412 14 |
0.95 (0.93–0.96) ⊕⊕⊕⊕ |
0.67 (0.59–0.75) ⊕⊕◯◯ |
Sensibility: / Specificity: -1 heterogeneity, -1 precision |
| SR (according to IOTA guidelines, varying handling of in-conclusive results) |
4795 10 |
0.89 (0.85–0.93) ⊕⊕⊕◯ |
0.86 (0.83–0.89) ⊕⊕⊕◯ |
Sensibility and Specificity: -1 combination of heterogeneity and precision |
| PR (ultrasound performed by an experienced sonographer) |
3187 6 |
0.93 (0.87–0.97) ⊕⊕⊕◯ |
0.83 (0.78–0.88) ⊕⊕⊕◯ |
Sensibility and Specificity: -1 combination of heterogeneity and precision |
The point estimates for PR, SR, RMI, and ROMA were clustered around 0.9, for sensitivity and around one-tenth lower for the specificity, it was not possible to statistically separate them. Both ADNEX and LR2 had a point estimate for sensitivity that was comparable in relation to the others but with a lower specificity. ADNEX was indistinguishable PR and RMI. The number of observations for LR2 was insufficient calculate a confidence region.
Ethics
There are several ethical aspects to consider when assessing the preoperative risk of ovarian cancer, such as the risks of overdiagnosis or underdiagnosis, invasion of privacy and integrity, equal access to care and the associated costs.
The ethical considerations in this report should be understood in the context of Swedish legislation and the fact that Swedish healthcare is largely publicly funded.
Discussion
Our assessment indicated that no algorithms surpassed the diagnostic accuracy of SR and PR, currently the most used methods in Sweden. The findings were derived from extensive data from multiple studies characterized by high observations. The studies primarily focused on specialized care settings with elevated cancer prevalence. The RMI algorithm, the most established, provided high specificity and negative predictive value, allowing for confidence in negative results. Notably, both ultrasound methodologies, SR and PR, along with RMI, exhibited high specificity in the premenopausal group, reinforcing the notion that negative results likely indicate benign cause, especially in low-prevalence scenarios with a high negative predictive value. The results for SR and PR indicate that sonographers found it easier to discern benign changes in premenopausal women compared to cancer, influenced by the understanding of lower cancer risk in this population. Both PR and SR present limitations, with PR necessitating a skilled and experienced sonographer, and IOTA certification ensures competency in examinations. SR is challenged by a substantial number of uncertain findings, approximating 20 percent. Different studies adopt varied approaches to manage these ambiguous cases. Most institutions pursue further diagnostics for uncertain SR findings, typically utilizing PR or imaging modalities like CT or MRI, while some classify these cases as malignant. Other facilities opt for structured follow-up with repeat ultrasound and CA125 testing within 8 to 12 weeks until malignancy risk is assessed, or the patient is deemed healthy. Implementing structured follow-ups may mitigate the incidence of suspected false positives and ambiguous results.
Conflict of Interest
In accordance with SBU’s requirements, the experts and scientific reviewers participating in this project have submitted statements about conflicts of interest. These documents are available at SBU’s secretariat. SBU has determined that the conditions described in the submissions are compatible with SBU’s requirements for objectivity and impartiality.
The report in Swedish
Project group
Experts
- Ellika Andolf, MD, Professor Emerita, Department of Clinical Science, Karolinska Institute, Stockholm, Danderyds Hospital
- Christer Borgfeldt, Professor, Senior Consultant, Linköping University, Linköping Hospital
From SBU
- Jan Holst, project director
- Sigurd Vitols, assistant project director
- Ann Kristine Jonsson, information specialsit until 1 February 2025
- Maja Kärrman Fredriksson, information specialist from 18 February 2025
- Johanna Wiss, health economist
- Elisabeth Gustafsson, project administrator until 20 september 2024
- Anna Attergren Granath, project administrator from 20 september 2024
- Jenny Odeberg, head of department
- Anna Levinsson, analyst
- Lotta Ryk, analyst
- Fredrik Tholander, analyst
Flow chart
References
- SBU. Vätskebiopsi vid diagnostik av äggstockscancer. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2024. SBU Bereder 376. [accessed Jun 3 2025]. Available from: https://www.sbu.se/376
- Broqvist M, Sandman L, Widenlou Nordmark A, Edin U. Nationell modell för öppna prioriteringar inom hälso- och sjukvård: ett verktyg för rangordning. Linköping: Linköping University Electronic Press; 2017. 2017:2. [accessed June 1 2025]. Available from: https://liu.diva-portal.org/smash/get/diva2:1144043/FULLTEXT01.pdf
- Svenska Kvalitetsregistret för Gynekologisk Cancer (SQRGC). Rapporten är baserat på datauttag från SQRGC: 2025-05-06: Statistik Incanet. Årsrapport 2024. Available from: https://statistik.incanet.se/gyncancer/
- RCC. Äggstockscancer med epitelial histologi. Nationellt vårdprogram Regionala cancercentrum i samverkan; 2025. [updated April 22 2025; accessed June 2 2025]. Available from: https://kunskapsbanken.cancercentrum.se/globalassets/cancerdiagnoser/gynekologi/aggstockscancer/nationellt-vardprogram-aggstockscancer.pdf
- RCC. Äggstockscancer icke-epitelial. Könscellstumörer och könssträngs-stromacellstumörer samt ovanliga icke-epiteliala tumörer. Nationellt vårdprogram. Regionala cancercentrum i samverkan; 2025. [updated Feb 25 2025].
- Leandersson P, Hogberg T, Dickman PW, Malander S, Borgfeldt C. Incidence and survival of epithelial ovarian, fallopian tube, peritoneal, and undesignated abdominal/pelvic cancers in Sweden 1960–2014: A population-based cohort study. BMC Cancer. 2021;21(1):465. Available from: https://doi.org/10.1186/s12885-021-08169-w
- Svenska Kvalitetsregistret för Gynekologisk Cancer (SQRGC). Svenska Kvalitetsregistret för Gynekologisk cancer (SQRGC). Årsrapport 2024. Rapporten är baserat på datauttag från SQRGC: 2025-09-10. Available from: https://statistik.incanet.se/gyncancer/
- Bankhead CR, Kehoe ST, Austoker J. Symptoms associated with diagnosis of ovarian cancer: a systematic review. BJOG: An International Journal of Obstetrics & Gynaecology. 2005;112(7):857-65. Available from: https://doi.org/https://doi.org/10.1111/j.1471-0528.2005.00572.x
- RCC. Nationellt vårdprogram äggstockscancer. 2023. Available from: https://cancercentrum.se/diagnosbehandling/cancerdiagnoser/gynekologiskacancersjukdomar/aggstock/vardprogram.7275.html
- Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164(5):1511-8. Available from: https://doi.org/10.1016/s0002-9440(10)63708-x
- Cho KR, Shih I-M. Ovarian Cancer. Annual Review of Pathology: Mechanisms of Disease. 2009;4(Volume 4, 2009):287-313. Available from: https://doi.org/https://doi.org/10.1146/annurev.pathol.4.110807.092246
- Wilson JMG, Jungner G, World Health O. Principles and practice of screening for disease. Geneva: World Health Organization; 1968.
- Henderson JT, Webber EM, Sawaya GF. Screening for Ovarian Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama. 2018;319(6):595-606. Available from: https://doi.org/10.1001/jama.2017.21421
- Rosenthal AN, Fraser LSM, Philpott S, Manchanda R, Burnell M, Badman P, et al. Evidence of Stage Shift in Women Diagnosed With Ovarian Cancer During Phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35(13):1411-20. Available from: https://doi.org/10.1200/jco.2016.69.9330
- Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian Cancer Prevention and Screening. Obstet Gynecol. 2018;131(5):909-27. Available from: https://doi.org/10.1097/aog.0000000000002580
- Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182-93. Available from: https://doi.org/10.1016/s0140-6736(21)00731-5
- IOTA. IOTA + Ultrasound Education. International Ovarian Tumor Analysis (IOTA) Group [accessed Oct 07]. Available from: https://iotaplus.org/en
- Hutchon D. The Risk of Malignancy in ovarian cancer calculator - decision support system. 2002. Available from: http://www.hutchon.net/RMIcalc.htm
- Barrenada L, Ledger A, Dhiman P, Collins G, Wynants L, Verbakel JY, et al. ADNEX risk prediction model for diagnosis of ovarian cancer: systematic review and meta-analysis of external validation studies. BMJ medicine. 2024;3(1):e000817. Available from: https://doi.org/https://dx.doi.org/10.1136/bmjmed-2023-000817
- Timmerman D, Ameye L, Fischerova D, Epstein E, Melis GB, Guerriero S, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. Bmj. 2010;341:c6839. Available from: https://doi.org/10.1136/bmj.c6839
- Valentin L, Ameye L, Savelli L, Fruscio R, Leone FP, Czekierdowski A, et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: logistic regression models do not help. Ultrasound Obstet Gynecol. 2011;38(4):456-65. Available from: https://doi.org/10.1002/uog.9030
- Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecologic Oncology. 2009;112(1):40-6. Available from: https://doi.org/https://doi.org/10.1016/j.ygyno.2008.08.031
- Li F, Tie R, Chang K, Wang F, Deng S, Lu W, et al. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and CA125 in predicting epithelial ovarian cancer: a meta-analysis. BMC cancer. 2012;12:258. Available from: https://doi.org/https://dx.doi.org/10.1186/1471-2407-12-258
- Kalapotharakos G, Asciutto C, Henic E, Casslén B, Borgfeldt C. High preoperative blood levels of HE4 predicts poor prognosis in patients with ovarian cancer. J Ovarian Res. 2012;5(1):20. Available from: https://doi.org/10.1186/1757-2215-5-20
- Timmerman D, Valentin L, Bourne TH, Collins WP, Verrelst H, Vergote I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound in Obstetrics & Gynecology. 2000;16(5):500-5. Available from: https://doi.org/https://doi.org/10.1046/j.1469-0705.2000.00287.x
- SBU. Utvärdering av metoder i hälso- och sjukvården och insatser i socialtjänsten: en metodbok. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2020. Available from: https://www.sbu.se/sv/metod/metodboken-2023/
- The Prisma 2020 Statement. BMJ 2021;372:n71. 2021. Available from: https://doi.org/http://dx.doi.org/10.1136/bmj.n71
- Davenport C, Rai N, Sharma P, Deeks JJ, Berhane S, Mallett S, et al. Menopausal status, ultrasound and biomarker tests in combination for the diagnosis of ovarian cancer in symptomatic women. The Cochrane database of systematic reviews. 2022;7:CD011964. Available from: https://doi.org/https://dx.doi.org/10.1002/14651858.CD011964.pub2
- The EndNote Team. EndNote 21. Philadelphia, PA: Clarivate; 2013.
- Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240-3. Available from: https://doi.org/10.3163/1536-5050.104.3.014
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. 2016. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27919275
- Whiting P, Rutjes A, Westwood M, Mallett S, Deeks J, Reitsma J, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155(8):529-36.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924. Available from: https://doi.org/10.1136/bmj.39489.470347.AD
- SBU. Etiska aspekter på insatser inom hälso- och sjukvården. En vägledning för att identifiera relevanta etiska aspekter. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2021. [accessed June 3 2025]. Available from: https://www.sbu.se/globalassets/ebm/etiska_aspekter_halso_sjukvarden.pdf
- Cochrane Central Register of Controlled Trials. Cochrane Library. [accessed Oct 8 2025]. Available from:https://www.cochranelibrary.com/central
- Sundar S, Agarwal R, Davenport C, Scandrett K, Johnson S, Sengupta P, et al. Risk-prediction models in postmenopausal patients with symptoms of suspected ovarian cancer in the UK (ROCkeTS): a multicentre, prospective diagnostic accuracy study. Lancet Oncol. 2024;25(10):1371-86. Available from: https://doi.org/https://dx.doi.org/10.1016/S1470-2045(24)00406-6
- Norwegian Register for Scientific Journals Series and Publishers. The Norwegian Directorate for Higher Education and Skills. Bergen. [updated March 6 2024; accessed April 23 2025]. Available from: https://kanalregister.hkdir.no/en/informasjonsartikler/search-in-the-register
- Van Calster B, Valentin L, Froyman W, Landolfo C, Ceusters J, Testa AC, et al. Validation of models to diagnose ovarian cancer in patients managed surgically or conservatively: multicentre cohort study. BMJ. 2020;370:m2614. Available from: https://doi.org/10.1136/bmj.m2614
- Landolfo C, Ceusters J, Valentin L, Froyman W, Van Gorp T, Heremans R, et al. Comparison of the ADNEX and ROMA risk prediction models for the diagnosis of ovarian cancer: a multicentre external validation in patients who underwent surgery. British journal of cancer. 2024;130(6):934-40. Available from: https://doi.org/https://dx.doi.org/10.1038/s41416-024-02578-x
- Abdalla N, Piórkowski R, Stanirowski P, Cendrowski K, Sawicki W. Can Replacing CA125 with HE4 in Risk of Malignancy Indices 1-4 Improve Diagnostic Performance in the Presurgical Assessment of Adnexal Tumors? Biomed Res Int. 2017;2017:6712376. Available from: https://doi.org/10.1155/2017/6712376
- Al Musalhi K, Al Kindi M, Al Aisary F, Ramadhan F, Al Rawahi T, Al Hatali K, et al. Evaluation of HE4, CA-125, Risk of Ovarian Malignancy Algorithm (ROMA) and Risk of Malignancy Index (RMI) in the Preoperative Assessment of Patients with Adnexal Mass. Oman medical journal. 2016;31(5):336-44. Available from: https://doi.org/https://dx.doi.org/10.5001/omj.2016.68
- Borges AL, Brito M, Ambrósio P, Condeço R, Pinto P, Ambrósio B, et al. Prospective external validation of IOTA methods for classifying adnexal masses and retrospective assessment of two-step strategy using benign descriptors and ADNEX model: Portuguese multicenter study. Ultrasound in Obstetrics & Gynecology. 2024;64(4):538-49. Available from: https://doi.org/https://doi.org/10.1002/uog.27641
- Ertas S, Vural F, Vural F, Tufekci EC, Ertas AC, Kose G, et al. Predictive Value of Malignancy Risk Indices for Ovarian Masses in Premenopausal and Postmenopausal Women. Asian Pacific journal of cancer prevention : APJCP. 2016;17(4):2177-83. Available from: https://doi.org/https://dx.doi.org/10.7314/apjcp.2016.17.4.2177
- Janas L, Stachowiak G, Glowacka E, Piwowarczyk I, Kajdos M, Soja M, et al. The use of CA125, human epididymis protein 4 (HE4), risk of ovarian malignancy algorithm (ROMA), risk of malignancy index (RMI) and subjective assessment (SA) in preoperative diagnosing of ovarian tumors. Ginekol Pol. 2024;95(5):321-7. Available from: https://doi.org/10.5603/GP.a2022.0144
- Krascsenits G, Balázs B, Dudnyikova A, Purcsi K, Orosz E, Pete I. [Investigating the predictive value of RMI and ROMA indices in patients with ovarian tumors of uncertain dignity]. Magy Onkol. 2016;60(4):320-7.
- Liest A-L, Omran AS, Mikiver R, Rosenberg P, Uppugunduri S. RMI and ROMA are equally effective in discriminating between benign and malignant gynecological tumors: A prospective population-based study. Acta obstetricia et gynecologica Scandinavica. 2019;98(1):24-33. Available from: https://doi.org/https://dx.doi.org/10.1111/aogs.13462
- Lycke M, Kristjansdottir B, Sundfeldt K. A multicenter clinical trial validating the performance of HE4, CA125, risk of ovarian malignancy algorithm and risk of malignancy index. Gynecologic oncology. 2018;151(1):159-65. Available from: https://doi.org/https://dx.doi.org/10.1016/j.ygyno.2018.08.025
- Meys EMJ, Jeelof LS, Achten NMJ, Slangen BFM, Lambrechts S, Kruitwagen RFPM, et al. Estimating risk of malignancy in adnexal masses: external validation of the ADNEX model and comparison with other frequently used ultrasound methods. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2017;49(6):784-92. Available from: https://doi.org/https://dx.doi.org/10.1002/uog.17225
- Niemi RJ, Saarelainen SK, Luukkaala TH, Maenpaa JU. Reliability of preoperative evaluation of postmenopausal ovarian tumors. Journal of ovarian research. 2017;10(1):15. Available from: https://doi.org/https://dx.doi.org/10.1186/s13048-017-0309-4
- Radosa MP, Camara O, Vorwergk J, Diebolder H, Winzer H, Mothes A, et al. Preoperative multimodal strategies for risk assessment of adnexal masses: analysis of 1362 cases in a gynecologic cancer center. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2011;21(6):1056-62. Available from: https://doi.org/https://dx.doi.org/10.1097/IGC.0b013e3182187eb0
- Radwan AM, Taema MI. Accuracy of the risk of malignancy index-I in diagnosing ovarian malignancy in menopausal women. Przeglad menopauzalny = Menopause review. 2023;22(1):1-5. Available from: https://doi.org/https://dx.doi.org/10.5114/pm.2023.126435
- Spagnol G, Marchetti M, Carollo M, Bigardi S, Tripepi M, Facchetti E, et al. Clinical Utility and Diagnostic Accuracy of ROMA, RMI, ADNEX, HE4, and CA125 in the Prediction of Malignancy in Adnexal Masses. Cancers. 2024;16(22). Available from: https://doi.org/10.3390/cancers16223790
- Testa A, Kaijser J, Wynants L, Fischerova D, Van Holsbeke C, Franchi D, et al. Strategies to diagnose ovarian cancer: new evidence from phase 3 of the multicentre international IOTA study. British journal of cancer. 2014;111(4):680-8. Available from: https://doi.org/https://dx.doi.org/10.1038/bjc.2014.333
- van den Akker PAJ, Zusterzeel PLM, Aalders AL, Snijders MPLM, Samlal RAK, Vollebergh JHA, et al. Use of risk of malignancy index to indicate frozen section analysis in the surgical care of women with ovarian tumors. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2016;133(3):355-8. Available from: https://doi.org/https://dx.doi.org/10.1016/j.ijgo.2015.10.019
- Vilendecic Z, Radojevic M, Stefanovic K, Dotlic J, Likic Ladjevic I, Dugalic S, et al. Accuracy of IOTA Simple Rules, IOTA ADNEX Model, RMI, and Subjective Assessment for Preoperative Adnexal Mass Evaluation: The Experience of a Tertiary Care Referral Hospital. Gynecologic and obstetric investigation. 2023;88(2):116-22. Available from: https://doi.org/10.1159/000529355
- Wang R, Yang Z. Evaluating the risk of malignancy in adnexal masses: validation of O-RADS and comparison with ADNEX model, SA, and RMI. Ginekol Pol. 2023;94(10):799-806. Available from: https://doi.org/10.5603/GP.a2023.0019
- Chen X, Zhou H, Chen R, He J, Wang Y, Huang L, et al. Development of a multimarker assay for differential diagnosis of benign and malignant pelvic masses. Clinica Chimica Acta. 2015;440:57-63. Available from: https://doi.org/https://doi.org/10.1016/j.cca.2014.11.013
- Chudecka-Glaz AM. ROMA, an algorithm for ovarian cancer. Clinica chimica acta; international journal of clinical chemistry. 2015;440:143-51. Available from: https://doi.org/https://dx.doi.org/10.1016/j.cca.2014.11.015
- Cradic KW, Lasho MA, Algeciras-Schimnich A. Validation of the Cut-points Recommended for ROMA Using the Roche Elecsys CA125 and HE4 Assays. Annals of clinical and laboratory science. 2018;48(1):90-3.
- Grenache DG, Heichman KA, Werner TL, Vucetic Z. Clinical performance of two multi-marker blood tests for predicting malignancy in women with an adnexal mass. Clinica chimica acta; international journal of clinical chemistry. 2015;438:358-63. Available from: https://doi.org/https://dx.doi.org/10.1016/j.cca.2014.09.028
- Kim B, Park Y, Kim B, Ahn HJ, Lee K-A, Chung JE, et al. Diagnostic performance of CA 125, HE4, and risk of Ovarian Malignancy Algorithm for ovarian cancer. Journal of clinical laboratory analysis. 2019;33(1):e22624. Available from: https://doi.org/10.1002/jcla.22624
- Molina R, Escudero JM, Auge JM, Filella X, Foj L, Torne A, et al. HE4 a novel tumour marker for ovarian cancer: comparison with CA 125 and ROMA algorithm in patients with gynaecological diseases. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2011;32(6):1087-95. Available from: https://doi.org/https://dx.doi.org/10.1007/s13277-011-0204-3
- Montagnana M, Danese E, Ruzzenente O, Bresciani V, Nuzzo T, Gelati M, et al. The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Clinical chemistry and laboratory medicine. 2011;49(3):521-5. Available from: https://doi.org/https://dx.doi.org/10.1515/CCLM.2011.075
- Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstetrics and gynecology. 2011;118(2 Pt 1):280-8. Available from: https://doi.org/https://dx.doi.org/10.1097/AOG.0b013e318224fce2
- Romagnolo C, Leon AE, Fabricio ASC, Taborelli M, Polesel J, Del Pup L, et al. HE4, CA125 and risk of ovarian malignancy algorithm (ROMA) as diagnostic tools for ovarian cancer in patients with a pelvic mass: An Italian multicenter study. Gynecol Oncol. 2016;141(2):303-11. Available from: https://doi.org/10.1016/j.ygyno.2016.01.016
- Shin KH, Kim HH, Kwon BS, Suh DS, Joo JK, Kim KH. Clinical Usefulness of Cancer Antigen (CA) 125, Human Epididymis 4, and CA72-4 Levels and Risk of Ovarian Malignancy Algorithm Values for Diagnosing Ovarian Tumors in Korean Patients With and Without Endometriosis. Annals of laboratory medicine. 2020;40(1):40-7. Available from: https://doi.org/https://dx.doi.org/10.3343/alm.2020.40.1.40
- Spacir Prskalo Z, Bulic P, Langer S, Gace M, Puljiz M, Danolic D, et al. Proofs for implementation of higher HE4 and ROMA index cut-off values in ovarian cancer preoperative stratification. J Obstet Gynaecol. 2019;39(2):195-201. Available from: https://doi.org/10.1080/01443615.2018.1476471
- Teh BH, Yong SL, Sim WW, Lau KB, Suharjono HN. Evaluation in the predictive value of serum human epididymal protein 4 (HE4), cancer antigen 125 (CA 125) and a combination of both in detecting ovarian malignancy. Hormone molecular biology and clinical investigation. 2018;35(1). Available from: https://doi.org/https://dx.doi.org/10.1515/hmbci-2018-0029
- Terlikowska KM, Dobrzycka B, Witkowska AM, Mackowiak-Matejczyk B, Sledziewski TK, Kinalski M, et al. Preoperative HE4, CA125 and ROMA in the differential diagnosis of benign and malignant adnexal masses. Journal of ovarian research. 2016;9(1):43. Available from: https://doi.org/https://dx.doi.org/10.1186/s13048-016-0254-7
- Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. British journal of cancer. 2011;104(5):863-70. Available from: https://doi.org/https://dx.doi.org/10.1038/sj.bjc.6606092
- Yang Y, Ju H, Huang Y. Diagnostic performance of IOTA SR and O-RADS combined with CA125, HE4, and risk of malignancy algorithm to distinguish benign and malignant adnexal masses. Eur J Radiol. 2023;165:110926. Available from: https://doi.org/10.1016/j.ejrad.2023.110926
- Zhang L, Chen Y, Wang K. Comparison of CA125, HE4, and ROMA index for ovarian cancer diagnosis. Current problems in cancer. 2019;43(2):135-44. Available from: https://doi.org/https://dx.doi.org/10.1016/j.currproblcancer.2018.06.001
- Zhang P, Wang C, Cheng L, Zhang P, Guo L, Liu W, et al. Comparison of HE4, CA125, and ROMA Diagnostic Accuracy: A Prospective and Multicenter Study for Chinese Women With Epithelial Ovarian Cancer. Medicine. 2015;94(52):e2402. Available from: https://doi.org/https://dx.doi.org/10.1097/MD.0000000000002402
- Zhu C, Zhang N, Zhong A, Xiao K, Lu R, Guo L. A combined strategy of TK1, HE4 and CA125 shows better diagnostic performance than risk of ovarian malignancy algorithm (ROMA) in ovarian carcinoma. Clinica chimica acta; international journal of clinical chemistry. 2022;524:43-50. Available from: https://doi.org/https://dx.doi.org/10.1016/j.cca.2021.11.018
- Elsner Hernández N, De Luis Escudero JF, Pérez Méndez LI, Báez Quintana DR, Bruno Santana E, Pérez Álvarez JA, et al. Evaluation of the incorporation of an IOTA-ADNEX model in the discrimination of adnexal masses in our third-level hospital centre, taking into account the menopausal status of patients. Five years of experience. Clinica e Investigacion en Ginecologia y Obstetricia. 2024;51(1). Available from: https://doi.org/10.1016/j.gine.2023.100910
- Spagnol G, Marchetti M, De Tommasi O, Vitagliano A, Cavallin F, Tozzi R, et al. Simple rules, O-RADS, ADNEX and SRR model: Single oncologic center validation of diagnostic predictive models alone and combined (two-step strategy) to estimate the risk of malignancy in adnexal masses and ovarian tumors. Gynecologic oncology. 2023;177:109-16. Available from: https://doi.org/10.1016/j.ygyno.2023.08.012
- Szubert S, Wojtowicz A, Moszynski R, Zywica P, Dyczkowski K, Stachowiak A, et al. External validation of the IOTA ADNEX model performed by two independent gynecologic centers. Gynecologic oncology. 2016;142(3):490-5. Available from: https://doi.org/https://dx.doi.org/10.1016/j.ygyno.2016.06.020
- Van Calster B, Van Hoorde K, Valentin L, Testa AC, Fischerova D, Van Holsbeke C, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ (Clinical research ed). 2014;349:g5920. Available from: https://doi.org/https://dx.doi.org/10.1136/bmj.g5920
- Yoeli-Bik R, Longman RE, Wroblewski K, Weigert M, Abramowicz JS, Lengyel E. Diagnostic Performance of Ultrasonography-Based Risk Models in Differentiating Between Benign and Malignant Ovarian Tumors in a US Cohort. JAMA network open. 2023;6(7):e2323289. Available from: https://doi.org/https://dx.doi.org/10.1001/jamanetworkopen.2023.23289
- Phinyo P, Patumanond J, Saenrungmuaeng P, Chirdchim W, Pipanmekaporn T, Tantraworasin A, et al. Diagnostic Added-Value of Serum CA-125 on the IOTA Simple Rules and Derivation of Practical Combined Prediction Models (IOTA SR X CA-125). Diagnostics (Basel). 2021;11(2). Available from: https://doi.org/10.3390/diagnostics11020173
- Piovano E, Cavallero C, Fuso L, Viora E, Ferrero A, Gregori G, et al. Diagnostic accuracy and cost-effectiveness of different strategies to triage women with adnexal masses: a prospective study. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2017;50(3):395-403. Available from: https://doi.org/https://dx.doi.org/10.1002/uog.17320
- Tian C, Han Y-W, Shi Z-J, Li Y-W, Xie L, Liu X-L, et al. Diagnostic value of the International Ovarian Tumor Analysis simple rules combined with contrast-enhanced ultrasound for adnexal masses. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2025;35(2):100049. Available from: https://doi.org/https://dx.doi.org/10.1016/j.ijgc.2024.100049
- Radosa MP, Vorwergk J, Fitzgerald J, Kaehler C, Schneider U, Camara O, et al. Sonographic discrimination between benign and malignant adnexal masses in premenopause. Ultraschall in der Medizin (Stuttgart, Germany : 1980). 2014;35(4):339-44. Available from: https://doi.org/https://dx.doi.org/10.1055/s-0033-1335728
- Van Gorp T, Veldman J, Van Calster B, Cadron I, Leunen K, Amant F, et al. Subjective assessment by ultrasound is superior to the risk of malignancy index (RMI) or the risk of ovarian malignancy algorithm (ROMA) in discriminating benign from malignant adnexal masses. European journal of cancer (Oxford, England : 1990). 2012;48(11):1649-56. Available from: https://doi.org/https://dx.doi.org/10.1016/j.ejca.2011.12.003
- Vural F, Aka N, Ertas S, Kose G, Tufekci EC. The ovarian cancers in geriatric population: the validity of inflammatory markers, malignancy risk indices 1, 2, 3, 4, and CA-125 levels in malignancy discrimination of adnexal masses. European journal of gynaecological oncology. 2016;37(6):846-51.
- Chan KKL, Chen C-A, Nam J-H, Ochiai K, Wilailak S, Choon A-T, et al. The use of HE4 in the prediction of ovarian cancer in Asian women with a pelvic mass. Gynecologic oncology. 2013;128(2):239-44. Available from: https://doi.org/https://dx.doi.org/10.1016/j.ygyno.2012.09.034
- Karlsen MA, Sandhu N, Høgdall C, Christensen IJ, Nedergaard L, Lundvall L, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecologic oncology. 2012;127(2):379‐83. Available from: https://doi.org/10.1016/j.ygyno.2012.07.106
- Li L, Wan J, Cai G, Yuan L, Liang J, Song J, et al. Value of serum human epididymis secretory protein 4 as a marker for differential diagnosis of malignant and benign gynecological diseases of patients in southern China. Clinica chimica acta; international journal of clinical chemistry. 2016;459:170-6. Available from: https://doi.org/https://dx.doi.org/10.1016/j.cca.2016.06.010
- Melo A, Verissimo R, Farinha M, Martins NN, Martins FN. Discriminative value of CA-125, HE4, Risk of Malignancy Index II (RMI-II) and Risk of Malignancy Algorithm (ROMA) in the differential diagnosis of pelvic masses: conclusions from a referral Centre in Portugal. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2018;38(8):1140-5. Available from: https://doi.org/https://dx.doi.org/10.1080/01443615.2018.1457632
- Novotny Z, Presl J, Kucera R, Topolcan O, Vrzalova J, Fuchsova R, et al. HE4 and ROMA index in Czech postmenopausal women. Anticancer research. 2012;32(9):4137-40.
- Ortiz-Muñoz B, Aznar-Oroval E, García García A, Covisa Peris A, Perez Ballestero P, Sanchez Yepes M, et al. HE4, Ca125 and ROMA algorithm for differential diagnosis between benign gynaecological diseases and ovarian cancer. Tumour Biol. 2014;35(7):7249-58. Available from: https://doi.org/10.1007/s13277-014-1945-6
- Park H, Shin JE, Lee DW, Kim MJ, Lee HN. Diagnostic Accuracy of the Risk of Ovarian Malignancy Algorithm in Clinical Practice at a Single Hospital in Korea. Annals of laboratory medicine. 2019;39(3):252-62. Available from: https://doi.org/https://dx.doi.org/10.3343/alm.2019.39.3.252
- Spacir Prskalo Z, Gaće M, Dobrijević S, Mayer L. Benefits human epidydimis protein (HE4) compared to traditional used tumor markers in gynecological oncology. Libri Oncologici [Internet]. 2015 [pristupljeno 18.06.2025.];43(1-3):9-14. Dostupno na: https://hrcak.srce.hr/189799. 2025.
- Salim E, Zubairi AM, Danish SH, Ali U. Diagnostic Accuracy of Risk of Ovarian Malignancy Algorithm (ROMA) in Post-Menopausal Patients with Ovarian Mass. J Coll Physicians Surg Pak. 2018;28(6):440-4. Available from: https://doi.org/10.29271/jcpsp.2018.06.440
- Shen F, Lu S, Peng Y, Yang F, Chen Y, Lin Y, et al. Performance of ROMA based on Architect CA 125 II and HE4 values in Chinese women presenting with a pelvic mass: A multicenter prospective study. Clin Chim Acta. 2017;471:119-25. Available from: https://doi.org/10.1016/j.cca.2017.05.029
- Nätverket mot gynekologisk cancer. Samhällskostnader för ovarialcancer 2018. 2020. Available from: https://gyncancer.se/rapportaggstockscancer/hela-rapporten-svenska/
- SFS 2017:30. Hälso- och sjukvårdslag. författningssamling S. Stockholm: Riksdagen. [accessed Oct 9 2025].
- Riksdagsskrivelse 1996/1997:185. Prioriteringar inom vården. Stockholm: Riksdagen. [accessed Oct 9 2025].
- SFS 2010:659. Patientsäkerhetslag. författningssamling S. Stockholm: Riksdagen. [accessed Oct 9 2025].
- SFS 2014:821. Patientlag. författningssamling S. Stockholm: Riksdagen. [accessed Oct 9 2025].
- Froyman W, Landolfo C, De Cock B, Wynants L, Sladkevicius P, Testa AC, et al. Risk of complications in patients with conservatively managed ovarian tumours (IOTA5): a 2-year interim analysis of a multicentre, prospective, cohort study. The Lancet Oncology. 2019;20(3):448-58. Available from: https://doi.org/10.1016/S1470-2045(18)30837-4
- Landolfo C, Bourne T, Froyman W, Van Calster B, Ceusters J, Testa AC, et al. Benign descriptors and ADNEX in two-step strategy to estimate risk of malignancy in ovarian tumors: retrospective validation in IOTA5 multicenter cohort. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2023;61(2):231-42. Available from: https://doi.org/https://dx.doi.org/10.1002/uog.26080
- Moro F, Momi M, Ledger A, Barrenada L, Ceusters J, Sturla D, et al. External validation of ultrasound-based models for differentiating between benign and malignant adnexal masses: a nationwide prospective multicenter study (IOTA phase 6). Am J Obstet Gynecol. 2025. Available from: https://doi.org/10.1016/j.ajog.2025.07.017

 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services
 Share on Facebook
Share on Facebook
 Share on LinkedIn
Share on LinkedIn
 Share via Email
Share via Email