Newborn screening for Metachromatic Leukodystrophy
A systematic Review
Main message
There is insufficient evidence to evaluate the accuracy of a screening test for metachromatic leukodystrophy (MLD) in newborns. Likewise, there is insufficient evidence to determine the efficacy of gene therapy using autologous stem cells to treat MLD.
Results
Based on the systematic search conducted in December 2024, we found:
- A screening test for MLD appears able to detect most MLD cases within a newborn population. However, more studies are required to evaluate its accuracy.
- There is insufficient evidence to evaluate the effectiveness of gene therapy using autologous stem cells on motor skills, cognitive or social function in MLD patients, as only one relevant study was included.
Aim and background
The aim of this systematic review was to evaluate the scientific literature regarding MLD screening tests for newborns and gene therapy for MLD patients, to support the National Board of Health and Welfare in their development of newborn screening programs.
MLD is a very rare inherited disorder that affects the central nervous system, leading to loss of cognitive and motor skills and eventually death. There are four different forms of the disease: late infantile, early juvenile, late juvenile and adult form. The first two forms present at an early age and progress more rapidly (particularly the late infantile form) than the late juvenile or adult forms. The effectiveness of treatment depends on early detection, preferably already pre-symptomatic. Gene therapy has been available for pre-symptomatic late infantile and pre-symptomatic or early symptomatic early juvenile MLD for the last few years, but not for the late juvenile or adult forms of MLD. As treatment is indicated for pre-symptomatic and early forms of MLD, distinguishing between the different forms prior to symptom onset is essential, justifying the evaluation of screening. Therefore, this report also includes a description of the natural course of MLD and challenges in predicting the different forms of MLD in a potential screening scenario.
Method
We conducted a systematic review and reported it in accordance with the PRISMA statement, to answer research questions pertaining to two of the National Board of Health and Welfare´s criteria for newborn screening programs. These questions were included in the systematic review (see PICO and PIRO below). Additionally, we presented a background on predicting the different forms of MLD based on experts included in the project and relevant literature identified in the search.
Inclusion criteria
PIRO
Population: Newborn children
Index test: Screening test (relevant to Swedish conditions) using dried blood spots in a two- or three-tier screening strategy; sulfatide levels followed by ARSA enzyme activity, with or without gene sequencing
Reference test: Clinical diagnosis, including confirmatory gene sequencing
Outcome: Diagnostic accuracy
Study design: Systematic reviews or quantitative primary studies
PICO
Population: Children with presumed late infantile or early juvenile MLD (in studies with mixed populations, only participants where forms differentiation was possible were included)
Intervention: Hematopoietic stem cell transplantation with gene therapy (HSCT-GT) or allogenic hematopoietic stem cell transplantation (HSCT)
Control: No intervention or other intervention aimed at treating the disease
Outcome: Mortality, motor skills, cognitive function and social function
Study design: Prospective or retrospective controlled studies
Language: English and Scandinavian languages
Databases searched: Cochrane Library (Wiley), Embase (Elsevier) and Medline (OvidSP), from 1990 to December 2024
Result
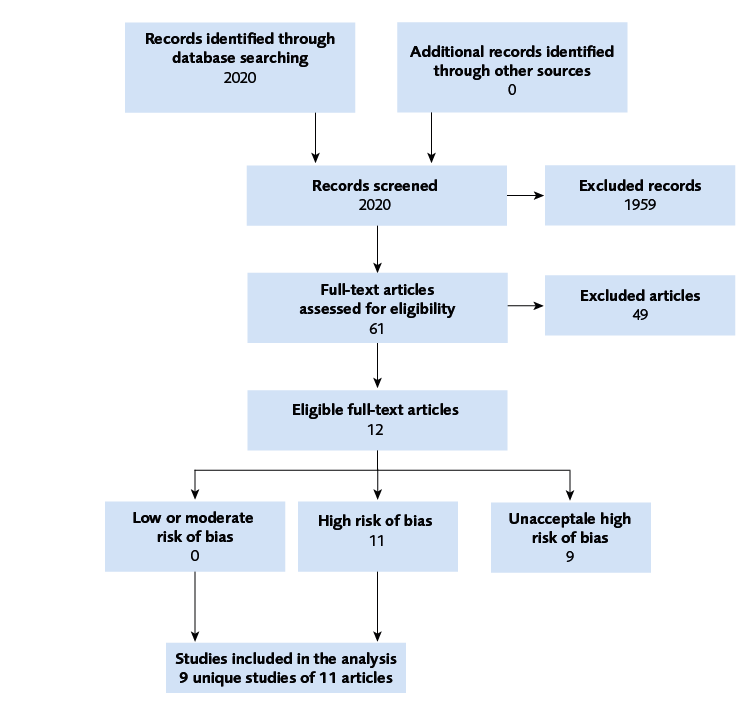
We identified three studies concerning screening tests, one study (with three publications) on HSCT-GT, and six studies on allogenic HSCT, one of which was excluded due to unacceptably high risk of bias (see Figure 1 for study flowchart).
No data synthesis or certainty of evidence rating was performed due to the small number of studies and large heterogeneity among included studies. We judge therefore that there is insufficient evidence to determine whether the screening tests or treatment are effective. However, a search for study protocols identified several ongoing studies that may be valuable for this topic further on.
Discussion
This report summarizes the evidence regarding newborn screening for MLD and its implications. The paucity of research is to be expected in a field where the disease is rare, and newborn screening for MLD is only fully implemented in one country but under consideration in others. Other systematic reviews of HCST-GT found similar results to those presented in this report. The challenge of accurately distinguishing between MLD forms in a newborn screening context must be considered when evaluating the implementation of a potential screening program.
Conflict of Interest
In accordance with SBU’s requirements, the experts and scientific reviewers participating in this project have submitted statements about conflicts of interest. These documents are available at SBU’s secretariat. SBU has determined that the conditions described in the submissions are compatible with SBU’s requirements for objectivity and impartiality.
The full report in Swedish
Project group
Experts
- Veroniqa Lundbäck, Clinical Scientist, PhD, Karolinska university hospital; Centre for Inherited Metabolic Diseases (CMMS) at Karolinska University Hospital, Stockholm
- Karin Naess, pediatric neurologist, Astrid Lindgren Children's Hospital and Center for Inherited Metabolic disorders (CMMS), Karolinska University Hospital, Stockholm
From SBU
- Fanny Sellberg, project director
- Martin Norman, assistant project director
- Jenny Ågren, analyst
- Carl Gornitzki, information specialist
- Emma Wernersson, project administrator
- Sigrid Widén, project administrator
- Jenny Odeberg, head of department
Flow chart
Appendices
- Search strategies (PDF)
- Excluded references (PDF)
- Risk of Bias in included studies (PDF)
- Characteristics of included studies (PDF)
References
- Laugwitz L, Schoenmakers DH, Adang LA, Beck-Woedl S, Bergner C, Bernard G, et al. Newborn screening in metachromatic leukodystrophy - European consensus-based recommendations on clinical management. Eur J Paediatr Neurol. 2024;49:141-54. Available from: https://doi.org/10.1016/j.ejpn.2024.03.003
- Hult M, Darin N, von Döbeln U, Månsson JE. Epidemiology of lysosomal storage diseases in Sweden. Acta Paediatr. 2014;103(12):1258-63. Available from: https://doi.org/10.1111/apa.12807
- Socialstyrelsen/ovanliga hälsotillstånd/Metakromatisk Leukodystrofi. Stockholm: Socialstyrelsen. Available from: https://www.socialstyrelsen.se/kunskapsstod-och-regler/omraden/sallsynta-halsotillstand/om-kunskapsdatabasen/sok-bland-sallsynta-halsotillstand/metakromatisk-leukodystrofi/
- University of Washington. GeneReviews. Seattle University of Washington; 1993. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1116/
- Cesani M, Lorioli L, Grossi S, Amico G, Fumagalli F, Spiga I, et al. Mutation Update of ARSA and PSAP Genes Causing Metachromatic Leukodystrophy. Hum Mutat. 2016;37(1):16-27. Available from: https://doi.org/10.1002/humu.22919
- ClinVar. National Library of Medicine. [accessed February 12 2025]. Available from: https://www.ncbi.nlm.nih.gov/clinvar/
- LOVD. Leiden University Medical Center. [accessed February 12 2025]. Available from: https://databases.lovd.nl/shared/genes/ARSA
- Elgun S, Waibel J, Kehrer C, van Rappard D, Bohringer J, Beck-Wodl S, et al. Phenotypic variation between siblings with Metachromatic Leukodystrophy. Orphanet J Rare Dis. 2019;14(1):136. Available from: https://doi.org/10.1186/s13023-019-1113-6
- Santhanakumaran V, Groeschel S, Harzer K, Kehrer C, Elgun S, Beck-Wodl S, et al. Predicting clinical phenotypes of metachromatic leukodystrophy based on the arylsulfatase A activity and the ARSA genotype? - Chances and challenges. Mol Genet Metab. 2022;137(3):273-82. Available from: https://doi.org/10.1016/j.ymgme.2022.09.009
- Lugowska A, Amaral O, Berger J, Berna L, Bosshard NU, Chabas A, et al. Mutations c.459+1G>A and p.P426L in the ARSA gene: prevalence in metachromatic leukodystrophy patients from European countries. Mol Genet Metab. 2005;86(3):353-9. Available from: https://doi.org/10.1016/j.ymgme.2005.07.010
- Bohringer J, Santer R, Schumacher N, Gieseke F, Cornils K, Pechan M, et al. Enzymatic characterization of novel arylsulfatase A variants using human arylsulfatase A-deficient immortalized mesenchymal stromal cells. Hum Mutat. 2017;38(11):1511-20. Available from: https://doi.org/10.1002/humu.23306
- Laugwitz L, Zizmare L, Santhanakumaran V, Cannet C, Bohringer J, Okun JG, et al. Identification of neurodegeneration indicators and disease progression in metachromatic leukodystrophy using quantitative NMR-based urinary metabolomics. JIMD rep. 2022;63(2):168-80. Available from: https://doi.org/10.1002/jmd2.12273
- Dubois G, Harzer K, Baumann N. Very low arylsulfatase A and cerebroside sulfatase activities in leukocytes of healthy members of metachromatic leukodystrophy family. Am J Hum Genet. 1977;29(2):191-4.
- Gieselmann V, Polten A, Kreysing J, von Figura K. Arylsulfatase A pseudodeficiency: loss of a polyadenylylation signal and N-glycosylation site. Proc Natl Acad Sci U S A. 1989;86(23):9436-40. Available from: https://doi.org/10.1073/pnas.86.23.9436
- Harvey JS, Carey WF, Morris CP. Importance of the glycosylation and polyadenylation variants in metachromatic leukodystrophy pseudodeficiency phenotype. Hum Mol Genet. 1998;7(8):1215-9. Available from: https://doi.org/10.1093/hmg/7.8.1215
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University School of Medicine. [accessed February 18 2025]. Available from: https://www.omim.org/entry/272200
- Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University School of Medicine. [accessed February 18 2025]. Available from: https://omim.org/entry/249900
- Covidence. Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation. Available from: www.covidence.org
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. Available from: https://doi.org/10.1136/bmj.i4919
- SBU. Manual till mallarna för randomiserade och icke randomiserade interventionsstudier. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2022. [updated May 11 2022]. Available from: https://www.sbu.se/globalassets/ebm/manual_mallarna_randomiserade_icke-randomiserade_kontrollerade_studier.pdf
- Hong X, Daiker J, Sadilek M, Ruiz-Schultz N, Kumar AB, Norcross S, et al. Toward newborn screening of metachromatic leukodystrophy: results from analysis of over 27,000 newborn dried blood spots. Genet Med. 2021;23(3):555-61. Available from: https://doi.org/10.1038/s41436-020-01017-5
- Laugwitz L, Mechtler TP, Janzen N, Oliva P, Kasper AR, Teunissen CE, et al. Newborn Screening and Presymptomatic Treatment of Metachromatic Leukodystrophy. N Engl J Med. 2024;391(13):1256-8. Available from: https://doi.org/10.1056/NEJMc2407165
- Wu THY, Brown HA, Church HJ, Kershaw CJ, Hutton R, Egerton C, et al. Improving newborn screening test performance for metachromatic leukodystrophy: Recommendation from a pre-pilot study that identified a late-infantile case for treatment. Mol Genet Metab. 2024;142(1):108349. Available from: https://doi.org/10.1016/j.ymgme.2024.108349
- Fumagalli F, Calbi V, Natali Sora MG, Sessa M, Baldoli C, Rancoita PMV, et al. Lentiviral haematopoietic stem-cell gene therapy for early-onset metachromatic leukodystrophy: long-term results from a non-randomised, open-label, phase 1/2 trial and expanded access. Lancet. 2022;399(10322):372-83. Available from: https://doi.org/10.1016/S0140-6736(21)02017-1
- Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):1233158. Available from: https://doi.org/10.1126/science.1233158
- Sessa M, Lorioli L, Fumagalli F, Acquati S, Redaelli D, Baldoli C, et al. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388(10043):476-87. Available from: https://doi.org/10.1016/S0140-6736(16)30374-9
- Boucher AA, Miller W, Shanley R, Ziegler R, Lund T, Raymond G, et al. Long-term outcomes after allogeneic hematopoietic stem cell transplantation for metachromatic leukodystrophy: the largest single-institution cohort report. Orphanet J Rare Dis. 2015;10:94. Available from: https://doi.org/10.1186/s13023-015-0313-y
- Chen X, Gill D, Shaw P, Ouvrier R, Troedson C. Outcome of Early Juvenile Onset Metachromatic Leukodystrophy After Unrelated Cord Blood Transplantation: A Case Series and Review of the Literature. J Child Neurol. 2016;31(3):338-44. Available from: https://doi.org/10.1177/0883073815595078
- Kasapkara ÇS, CİVELEK ÜREY B, BİLGİNER GÜRBÜZ B, Küçükçongar Yavaş A, Keçeli AM, Öncül Ü, et al. Clinical and Radiological Profile of Nine Patients with Metachromatic Leukodystrophy. Molecular Syndromology. 2024.
- Pridjian G, Humbert J, Willis J, Shapira E. Presymptomatic late-infantile metachromatic leukodystrophy treated with bone marrow transplantation. J Pediatr. 1994;125(5 Pt 1):755-8. Available from: https://doi.org/10.1016/s0022-3476(94)70072-9
- van Rappard DF, Boelens JJ, van Egmond ME, Kuball J, van Hasselt PM, Oostrom KJ, et al. Efficacy of hematopoietic cell transplantation in metachromatic leukodystrophy: the Dutch experience. Blood. 2016;127(24):3098-101. Available from: https://doi.org/10.1182/blood-2016-03-708479
- Guffon N, Souillet G, Maire I, Dorche C, Mathieu M, Guibaud P. Juvenile metachromatic leukodystrophy: neurological outcome two years after bone marrow transplantation. J Inherit Metab Dis. 1995;18(2):159-61. Available from: https://doi.org/10.1007/BF00711755
- ChiCTR-OPC-15005802. Autologous hematopoietic stem cell gene therapy for Metachromatic Leukodystrophy and Adrenoleukodystrophy. 2015.
- Dangouloff T, Boemer F. Baby Detect : Genomic Newborn Screening. ClinicalTrials.gov 2022 NCT05687474. [updated Feb 12 2025]. Available from: https://clinicaltrials.gov/ct2/show/NCT05687474
- Orchard P, Burke L. MT2013-31: Allo HCT for Metabolic Disorders and Severe Osteopetrosis. ClinicalTrials.gov 2014 NCT02171104. [updated Aug 3 2023; accessed Mar 5 2025]. Available from: https://clinicaltrials.gov/ct2/show/NCT02171104
- Orchard Therapeutics. A Safety and Efficacy Study of Cryopreserved OTL-200 for Treatment of Metachromatic Leukodystrophy (MLD). ClinicalTrials.gov 2017 NCT03392987. [updated Jan 27 2025; accessed Mar 5 2025]. Available from: https://clinicaltrials.gov/ct2/show/NCT03392987
- Orchard Therapeutics. OTL-200 in Patients With Late Juvenile Metachromatic Leukodystrophy (MLD). ClinicalTrials.gov 2020 NCT04283227. Available from: https://clinicaltrials.gov/ct2/show/NCT04283227
- Vanderver A. LeukoSEQ: Whole Genome Sequencing as a First-Line Diagnostic Tool for Leukodystrophies. ClinicalTrials.gov 2016 NCT02699190 [updated Jan 24 2025; accessed Mar 5 2025]. Available from: https://clinicaltrials.gov/ct2/show/NCT02699190
- Wasserstein M. ScreenPlus: A Comprehensive, Flexible, Multi-disorder Newborn Screening Program. ClinicalTrials.gov 2022 NCT05368038. [updated Sep 19 2024; accessed Mar 5 2025]. Available from: https://clinicaltrials.gov/show/NCT05368038
- Zhou J, Lian Q. A Phase I/II Clinical Trial of Lentiviral Hematopoietic Stem Cell Gene Therapy for Treatment of Developed Metachromatic Leukodystrophy and Adrenoleukodystrophy. 2015 NCT02559830. [updated May 31 2022; accessed Mar 5 2025]. Available from: https://clinicaltrials.gov/study/NCT02559830
- Armstrong N, Olaye A, Noake C, Pang F. A systematic review of clinical effectiveness and safety for historical and current treatment options for metachromatic leukodystrophy in children, including atidarsagene autotemcel. Orphanet J Rare Dis. 2023;18(1):248. Available from: https://doi.org/10.1186/s13023-023-02814-2
- Helsedirektoratet 2023:Vurdering av soknad om endring av nasjonal og flerregional behandlingstjeneste 2023. Helsedirekoratet. [accessed Mar 5 2025]. Available from: https://www.regjeringen.no/contentassets/33b9ed287add413aaa40dca6d7e533fd/nyfodt-direktoratets-vurderinger-31-jan-og-27-feb.pdf
- LOVDATA. Helse- og omsorgsdepartementet; 2024. Forskrift om endring i forskrift 29. juni 2007 nr. 742 om genetisk masseundersøkelse av nyfødte. Available from: https://lovdata.no/dokument/LTI/forskrift/2024-06-25-1223
- Medical C. Screening for Metachromatic Leukodystrophy. London: Department of Health and Social Care; 2023. [accessed February 19 2025]. Available from: https://view-health-screening-recommendations.service.gov.uk/document/626/download
- Committee UNS. Newborn screening programme Metachromatic leukodystrophy. GOV.UK; 2023. UK NSC Recommendations. [accessed February 18 2025]. Available from: https://view-health-screening-recommendations.service.gov.uk/metachromatic-leukodystrophy/

 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services
 Share on Facebook
Share on Facebook
 Share on LinkedIn
Share on LinkedIn
 Share via Email
Share via Email