Treatment of depression with transcranial magnetic stimulation using an H-coil (dTMS)
A systematic review and assessment of medical, economic, social and ethical aspects. An HTA Report
This report is part of a government assignment regarding mental illnesses and consists of a systematic review of therapeutic effects and adverse events in the treatment of depression with deep transcranial magnetic stimulation (dTMS), which is a variant of repetitive transcranial magnetic stimulation (rTMS).
Conclusions
- It is uncertain how the effects of dTMS compare with those of rTMS with a figure-of-8 coil. There are no reliable studies comparing dTMS with other treatments for depression.
- Four weeks of treatment with dTMS compared with treatment with a sham coil results in an 11% increase in remission (between 1% and 22%) in patients with depression who were previously treated with antidepressants (⊕⊕◯◯). The long-term effects of dTMS treatment are uncertain.
- Epileptic seizures can occur during dTMS treatment, but they are uncommon. There are no indications of cognitive side effects. Reports of local pain increases with 22% (between 15% and 28%) in patients treated with dTMS compared to patients treated with a sham coil (⊕⊕⊕◯).
The intention behind the development of dTMS was to achieve a larger effect than that observed with the established treatment using rTMS with a figure-of-8-coil in patients previously treated with antidepressants. The effects of dTMS in comparison with other treatments have not been sufficiently evaluated; however, the effect size seems to be similar to that achievable with rTMS using a different coil type.
The studies evaluating dTMS have included different populations and different numbers of previous treatment attempts. The possibility that dTMS is more effective for specific groups of patients cannot be excluded, and it would be valuable to perform studies focusing on well-defined patient groups that currently lack suitable alternative treatments.
Background
Alternative treatments are needed for patients with depression who do not improve after treatment with antidepressant drugs or psychological treatment. One alternative is rTMS, where an electromagnetic coil is placed on the patient’s head and repetitive pulses create a changing magnetic field that induces electric current that stimulates parts of the brain that are thought to be involved in the mechanisms of depression. dTMS is a variant of rTMS, in which a different shaped coil is used. The coil used in dTMS is referred to as an H-coil, whereas the most common coil type in rTMS is a figure-of-8 coil.
Aim
To assess the effects and adverse events of treating depression with dTMS. The questions asked were as follows:
- Does dTMS affect depression when the depressive state is assessed at the end of treatment or during follow-up sometime after the end of treatment?
- If dTMS has a statistically significant therapeutic effect, can maintenance treatment maintain this effect?
- What side effects and complications are there with dTMS treatment, and how common are they?
Method
This systematic review was conducted in accordance with the PRISMA statement. The protocol is registered in Prospero (CRD42020193623, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020193623). The certainty of evidence was assessed using GRADE (https://gdt.gradepro.org/app/handbook/handbook.html).
Inclusion criteria
Population: Patients with unipolar or bipolar depression according to DSM-III to DSM-5 or ICD-10 criteria.
Intervention: Transcranial magnetic stimulation with an H-coil.
Control: Sham coil, other treatments such as rTMS using a different coil type, ECT, or antidepressant drugs, or treatment with another dose of dTMS.
Outcome: Percentage of patients in remission, percentage of patients with a response or change in score on a depression-rating scale. Adverse events and complications of treatment.
Study design: Randomised controlled trials.
Search period: From 2014 to 2020. Final search on February 24, 2020.
Databases searched: Cochrane Library (Wiley), EMBASE (Embase.com), PsycINFO (EBSCO), PubMed (NLM).
A complementary search of Clinicaltrials.gov was performed in September 2020 for studies using dTMS for the treatment of depression.
Client/patient involvement: No.
Results
Five randomised clinical trials that described the effects and side effects of dTMS were included in this systematic review (Figure 1). These studies included 582 participants. The results of the assessment are summarised in Figure 1 and Table 1 below.
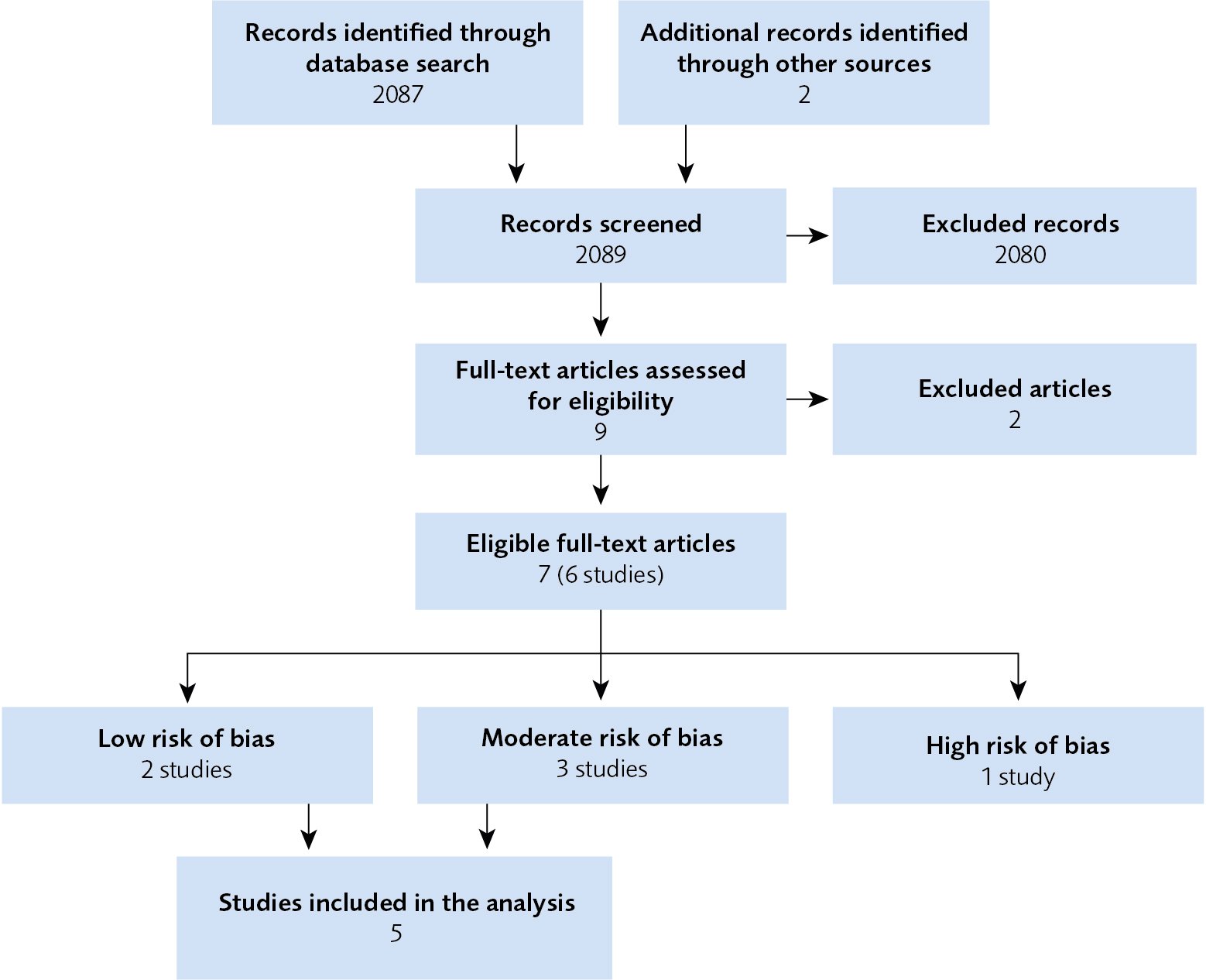
Figure 1 Flow chart of the article selection procedure.
| CI = Confidence interval; MD = Mean difference; NA = Not applicable; RD = Risk difference; RR = Risk ratio; SD = Standard deviation. 1 Deducion of 1 point for risk of bias due to uncertainties in the reporting, especially regarding the largest study, and because three of four studies were sponsored by the company that developed the product, and it is unclear how involved the company was in these studies. 2 Deduction of 1 point for precision as there were few participants and few events in the studies. 3 Deduction of 1 point for precision as there were few participants and studies. 4 Deduction of 3 points for precision as there was only one study, which had few participants. * Mean difference and standard deviation cannot be reported as the change in depression score was reported in different ways across the studies. |
|||||
| Outcome | Number of participants (Number of studies) Reference Study design | Result MD (SD) | Effect RR/RD or MD (95% CI) | Certainty of evidence | Deduction |
|---|---|---|---|---|---|
| dTMS compared with a sham coil at end of treatment | |||||
| Increase in the proportion achieving remission | 354 (4) [1-4] RCT |
dTMS: 0.26 (0.12) sham coil: 0.15 (0.01) |
RD: 0.11 (0.01 to 0.22) (RR: 1.88 (1.22 to 2.88)) |
Low ⊕⊕◯◯ |
–1 risk of bias1 –1 precision2 |
| Increase in the proportion achieving a response | 354 (4) [1-4] RCT |
dTMS: 0.34 (0.17) sham coil: 0.20 (0.03) |
RD: 0.13 (0.01 to 0.25) (RR: 1.72 (1.2 to 2.46)) |
Low ⊕⊕◯◯ |
–1 risk of bias1 –1 precision2 |
| Decrease in depression score | 354 (4) [1-4] RCT |
NA* | MD: –2.85 (–4.18 to −1.51) |
Low ⊕⊕◯◯ |
–1 risk of bias1 –1 precision3 |
| dTMS compared with rTMS with a figure-of-8 coil | |||||
| Increase in the proportion achieving remission | 147 (1) [5] RCT |
dTMS: 0.60 rTMS: 0.43 |
RD: 0.17 (0.01 to 0.33) (RR: 1.40 (1.01 to 1.93)) |
Very low ⊕◯◯◯ |
–3 precision4 |
| Increase in the proportion achieving a response | 147 (1) [5] RCT |
dTMS: 0.67 rTMS: 0.44 |
RD: 0.23 (0.07 to 0.38) (RR: 1.52 (1.12 to 2.05)) |
Very low ⊕◯◯◯ |
–3 precision4 |
| Decrease in depression score | 147 (1) [5] RCT |
dTMS: −10< rTMS: −7 |
MD: –3.00 (−5.03 to −0.97) |
Very low ⊕◯◯◯ |
–3 precision4 |
| dTMS compared with a sham coil 4 weeks after end of treatment | |||||
| Proportion in remission | 50 (1) [4] RCT |
dTMS: 0.24 sham coil: 0.24 |
RD: 0.00 (–0.24 to 0.24) (RR: 1.00 (0.37 to 2.68)) |
Very low ⊕◯◯◯ |
–3 precision4 |
| Increase in the proportion achieving a response | 50 (1) [4] RCT |
dTMS: 0.32 sham coil: 0.24 |
RD: 0.08 (–0.17 to 0.33) (RR: 1.33 (0.54 to 3.29)) |
Very low ⊕◯◯◯ |
–3 precision4 |
| Decrease in depression score | 50 (1) [4] RCT |
dTMS: −9.32 sham coil: −6.08 |
MD: –2.76 (–8.24 to 2.72) |
Very low ⊕◯◯◯ |
–3 precision4 |
| Adverse events of dTMS compared with a sham coil | |||||
| Increase in the proportion with experience of local pain at the application site | 335 (3) [1,4,6] RCT |
dTMS: 0.20 (0.04) sham coil: 0.00 (0.00) |
RD: 0.22 (0.15 to 0.28) (RR: 17.70 (4.30 to 72.81)) |
Moderate ⊕⊕⊕◯ |
–1 precision3 |
The full report in Swedish
The full report "Behandling av depression med transkraniell magnetstimulering med H-spole (dTMS)" (in Swedish)
References
- Kaster TS, Daskalakis ZJ, Noda Y, Knyahnytska Y, Downar J, Rajji TK, et al. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology 2018;43:2231-8.
- Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry 2015;14:64-73.
- Matsuda Y, Kito S, Igarashi Y, Shigeta M. Efficacy and Safety of Deep Transcranial Magnetic Stimulation in Office Workers with Treatment-Resistant Depression: A Randomized, Double-Blind, Sham-Controlled Trial. Neuropsychobiology 2020;79:208-13.
- Tavares DF, Myczkowski ML, Alberto RL, Valiengo L, Rios RM, Gordon P, et al. Treatment of Bipolar Depression with Deep TMS: Results from a Double-Blind, Randomized, Parallel Group, Sham-Controlled Clinical Trial. Neuropsychopharmacology 2017;42:2593-601.
- Filipčić I, Šimunović Filipčić I, Milovac Ž, Sučić S, Gajšak T, Ivezić E, et al. Efficacy of repetitive transcranial magnetic stimulation using a figure-8-coil or an H1-Coil in treatment of major depressive disorder; A randomized clinical trial. J Psychiatr Res 2019;114:113-9.
- U.S. Food and Drug Administration (FDA). 510(K) SUMMARY Brainsway Deep TMS System; 2013. [cited 2020 Sep 10]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf12/k122288.pdf
Project group
Experts
- Axel Nordenskjöld, Psychiatrist, Örebro University Hospital, Örebro
- Björn Mårtensson, Psychiatrist, Karolinska institutet, Stockholm
From SBU
- Susanne Johansson, Project Manager
- Agneta Pettersson, Assistant Project Manager
- Sara Fundell, Project Administrator
- Klas Moberg, Information Specialist
External reviewers
- Robert Bodén, Psychiatrist, Uppsala University Hospital, Uppsala
Appendices
- Search strategies (pdf)
- Characteristics of included studies (pdf)
- Risk of bias chart (pdf)
- Excluded studies (pdf)
Reference list of the full report
- SBU. Effekter av djup transkraniell magnetstimulering med H-spole vid depression. Stockholm: Statens beredning för medicinsk utvärdering (SBU); 2015. SBU Alert-rapport nr 2015-05. ISSN 1652-7151. https://www.sbu.se.
- SBU. Diagnostik och uppföljning av förstämningssyndrom. En systematisk litteraturöversikt. Stockholm: Statens beredning för medicinsk utvärdering (SBU); 2012. SBU-rapport nr 212. ISBN 978-91-85413-52-2.
- Mattisson C, Bogren M, Nettelbladt P, Munk-Jörgensen P, Bhugra D. First incidence depression in the Lundby Study: a comparison of the two time periods 1947-1972 and 1972-1997. J Affect Disord 2005;87:151-60.
- Rush AJ, Warden D, Wisniewski SR, Fava M, Trivedi MH, Gaynes BN, et al. STAR*D: revising conventional wisdom. CNS Drugs 2009;23:627-47.
- Voineskos D, Daskalakis ZJ, Blumberger DM. Management of Treatment-Resistant Depression: Challenges and Strategies. Neuropsychiatr Dis Treat 2020;16:221-34.
- Socialstyrelsen. Nationella riktlinjer för vård vid depression och ångestsyndrom - Stöd för styrning och ledning. Stockholm: Socialstyrelsen; 2020. Artikelnummer 2020-9-6936.
- FASS. Produktresumé Spravato. [cited 2020 Sep 30] Available from: https://www.fass.se/LIF/product?userType=0&nplId=20181012000036&docType=6&scrollPosition=0.
- Maj M, Stein DJ, Parker G, Zimmerman M, Fava GA, De Hert M, et al. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry 2020;19:269-93.
- van Diermen L, van den Ameele S, Kamperman AM, Sabbe BCG, Vermeulen T, Schrijvers D, et al. Prediction of electroconvulsive therapy response and remission in major depression: meta-analysis. Br J Psychiatry 2018;212:71-80.
- Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry 2010;68:568-77.
- SBU. Transkraniell magnetstimulering vid depression. En systematisk litteraturöversikt. Stockholm: Statens beredning för medicinsk utvärdering (SBU); 2009. SBU-rapport nr 192. ISBN 978-91-85413-29-4.
- Berlim MT, Van den Eynde F, Jeff Daskalakis Z. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology 2013;38:543-51.
- Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med 2014;44:225-39.
- Ren J, Li H, Palaniyappan L, Liu H, Wang J, Li C, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2014;51:181-9.
- Elvin T, Nordenskjöld A. Årsrapport 2019. Kvalitetsregister för repetitiv transkraniell magnetstimulering (rTMS). Kvalitetsregister ECT. Region Örebro län; [cited 2020 Sep 10]. Available from: https://registercentrum.blob.core.windows.net/ect/r/-rsrapport_rTMS_2019-BygLxj3Q38.pdf.
- Deng ZD, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol 2014;125:1202-12.
- Levkovitz Y, Harel EV, Roth Y, Braw Y, Most D, Katz LN, et al. Deep transcranial magnetic stimulation over the prefrontal cortex: evaluation of antidepressant and cognitive effects in depressive patients. Brain Stimul 2009;2:188-200.
- Filipčić I, Šimunović Filipčić I, Milovac Ž, Sučić S, Gajšak T, Ivezić E, et al. Efficacy of repetitive transcranial magnetic stimulation using a figure-8-coil or an H1-Coil in treatment of major depressive disorder; A randomized clinical trial. J Psychiatr Res 2019;114:113-9.
- Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry 2015;14:64-73.
- Matsuda Y, Kito S, Igarashi Y, Shigeta M. Efficacy and Safety of Deep Transcranial Magnetic Stimulation in Office Workers with Treatment-Resistant Depression: A Randomized, Double-Blind, Sham-Controlled Trial. Neuropsychobiology 2020;79:208-13.
- Tavares DF, Myczkowski ML, Alberto RL, Valiengo L, Rios RM, Gordon P, et al. Treatment of Bipolar Depression with Deep TMS: Results from a Double-Blind, Randomized, Parallel Group, Sham-Controlled Clinical Trial. Neuropsychopharmacology 2017;42:2593-601.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9, w64.
- SBU. SBU:s metodbok. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); [cited 2020 Oct 16]. Available from: https://www.sbu.se/metodbok/.
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210.
- Schünemann H, Brożek J, Gordon G, Oxman A. GRADE Handbook. Introduction to GRADE Handbook. Handbook for grading the qualityof evidence and the strength of recommendations using the GRADE approach. Updated October 2013; [cited 2020 Sep 10]. Available from: https://gdt.gradepro.org/app/handbook/handbook.html.
- Rapinesi C, Bersani FS, Kotzalidis GD, Imperatori C, Del Casale A, Di Pietro S, et al. Maintenance Deep Transcranial Magnetic Stimulation Sessions are Associated with Reduced Depressive Relapses in Patients with Unipolar or Bipolar Depression. Front Neurol 2015;6:16.
- Kaster TS, Daskalakis ZJ, Noda Y, Knyahnytska Y, Downar J, Rajji TK, et al. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology 2018;43:2231-8.
- Myczkowski ML, Fernandes A, Moreno M, Valiengo L, Lafer B, Moreno RA, et al. Cognitive outcomes of TMS treatment in bipolar depression: Safety data from a randomized controlled trial. J Affect Disord 2018;235:20-6.
- U.S. Food and Drug Administration (FDA). 510(K) SUMMARY Brainsway Deep TMS System; 2013. [cited 2020 Sep 10]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf12/k122288.pdf.
- Gellersen HM, Kedzior KK. Antidepressant outcomes of high-frequency repetitive transcranial magnetic stimulation (rTMS) with F8-coil and deep transcranial magnetic stimulation (DTMS) with H1-coil in major depression: a systematic review and meta-analysis. BMC Psychiatry 2019;19:139.
- Hung YY, Yang LH, Stubbs B, Li DJ, Tseng PT, Yeh TC, et al. Efficacy and tolerability of deep transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2020;99:109850.
- Zheng W, Cai DB, Xiang YQ, Zheng W, Jiang WL, Sim K, et al. Adjunctive intranasal esketamine for major depressive disorder: A systematic review of randomized double-blind controlled-placebo studies. J Affect Disord 2020;265:63-70.
- Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 2003;361:799-808.
- Repetitive Transcranial Magnetic Stimulation for Treatment-Resistant Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ont Health Technol Assess Ser 2016;16:1-66.
- Tendler A, Roth Y, Zangen A. Rate of inadvertently induced seizures with deep repetitive transcranial magnetic stimulation. Brain Stimul 2018;11:1410-4.
- Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, Available from www.training.cochrane.org/handbook.
- Carrozzino D, Patierno C, Fava GA, Guidi J. The Hamilton Rating Scales for Depression: A Critical Review of Clinimetric Properties of Different Versions. Psychother Psychosom 2020;89:133-50.
- Hieronymus F, Emilsson JF, Nilsson S, Eriksson E. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol Psychiatry 2016;21:523-30.

 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services
 Share on Facebook
Share on Facebook
 Share on LinkedIn
Share on LinkedIn
 Share via Email
Share via Email