This publication was published more than 5 years ago. The state of knowledge may have changed.
Vacuum Assisted Wound Closure Therapy
This report was produced in collaboration between SBU and the Regional HTA Centre of Region Västra Götaland.
SBU’s appraisal of the evidence
Vacuum assisted closure (VAC) therapy is a method intended to accelerate the healing of surgical wounds and wounds that fail to heal on their own (primary healing), e.g. after suturing. In recent years, Swedish hospitals have started to use this method to a greater extent.
Many controlled trials have been published that address the method’s effects on wound healing, length of hospital stay, and complications involving different types of wounds. Further, a smaller number of studies have been published regarding the effects on mortality. None are of high scientific quality, and only a few are of moderate scientific quality.
- The scientific documentation on vacuum assisted closure therapy offers some evidence that the method:
- yields faster healing and a higher percentage of healed wounds in patients with split-thickness skin grafts* for wounds that are not “surgically clean”, in patients with inflammation in the thoracic cavity (mediastinitis) following surgery where the sternum is divided (sternotomy), and in patients with diabetes where gangrene necessitates forefoot amputation.
- leads to fewer infections and fewer wound complications in patients with orthopaedic trauma and open fractures.
- leads to a shorter length of hospital stay for patients with split-thickness skin grafts* for wounds that are not “surgically clean”.
- reduces mortality in post-sternotomy patients with mediastinitis.
- The utility of the method is uncertain in many clinical situations. The review of the scientific documentation reveals a lack of well-executed studies involving patients with split-thickness skin grafts* in otherwise “healthy” wounds, with open abdominal wounds, with a necrotising fasciitis, with severe deep infection in the tissues between the urethra and the rectum (Fournier gangrene), with an open wound after fasciotomy, or with a tissue defect following musculoskeletal tumour surgery.
- Randomised, controlled trials of different well-defined wound types are urgently needed. There continues to be a lack of good-quality health economic assessments.
* Plastic surgery procedure where a section of the epidermis is removed and transplanted to another site on the body.
Technology and target group
Vacuum assisted closure therapy is used for many different types of wounds that require secondary healing (i.e. from the wound floor and edges). Reasons could be that the wound is infected, that the tissue near the wound is damaged or swollen, or that a healing wound has opened. Generally, patients with such wounds are severely ill and often require a long period of hospital care. Also, mortality is high in some categories of patients.
Vacuum assisted closure therapy requires a sealed and moist wound environment. The intent is that negative pressure in the wound will cause swelling to subside more quickly, that the wound will be cleansed more effectively, and that blood circulation in the wound region will increase. Hence, the wound healing processes (granulation) will accelerate, along with reformation of the outer (epithelial) layer of skin, so the wound will heal faster.
An advantage of vacuum assisted closure therapy is that usually the wound only needs to be dressed every second or third day instead of daily, as is the case with conventional treatment.
The method was first used in Sweden in the early years after 2000. Knowledge about its mechanism of action is based mainly on animal studies. In recent years, further controlled trials and case series have been published.
The patient groups that we analysed in this report are adult patients with surgical wounds after some type of intervention where primary wound healing cannot take place. However, we did not include patients with pressure sores or diabetes patients with wounds that have only been debrided surgically.
Primary questions
- Does VAC therapy after surgical intervention lead to faster and more effective wound healing (healing time, reduction of wound surface, skin graft healing time) compared to conventional wound treatment?
- Does VAC therapy after surgical intervention lead to shorter length of stay in hospital compared to conventional wound treatment?
- Does VAC therapy after surgical intervention lead to lower mortality compared to conventional wound treatment?
- What side effects or complications are associated with VAC therapy?
- What does VAC therapy cost? What is its cost-effectiveness?
Patient benefit
- There is some evidence that VAC therapy yields better healing of transplanted skin and a shorter length of stay than conventional wound treatment in patients that receive split-thickness skin grafts because the skin in direct contact with the wound provides insufficient coverage due to trauma, burns, infection, or pressure (low quality evidence, GRADE
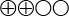 ).
). - There is some evidence that VAC therapy yields fewer infections and wound complications than conventional wound treatment in patients with wounds following orthopaedic trauma and open fractures (low quality evidence, GRADE
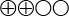 ).
). - There is some evidence that VAC therapy yields better wound healing, a shorter length of stay, and lower hospital mortality than conventional wound treatment in patients with mediastinitis and unsuccessful wound healing following sternotomy (low quality evidence, GRADE
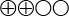 ).
). - There is some evidence that VAC therapy improves wound healing in comparison to conventional wound treatment in patients with diabetes mellitus and gangrene that necessitates amputation (low quality evidence, GRADE
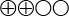 ).
). - Scientific documentation is lacking or insufficient (very low quality evidence, GRADE
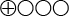 ) regarding the effects of VAC therapy in the following categories:
) regarding the effects of VAC therapy in the following categories:
- Patients with split-thickness skin grafts in an otherwise “healthy” wound
- Patients with open abdominal wounds
- Patients with necrotising fasciitis
- Patients with Fournier gangrene
- Patients with open wounds after fasciotomy
- Patients with tissue defects following musculoskeletal tumour surgery
Ethical aspects
An ethical dilemma could arise if a health care provider does not offer VAC therapy, and the clinician asserts that the patient is being denied a beneficial, non-dangerous therapy that involves fewer dressing changes and less-offensive odour. On the other hand, one must question whether it is defensible to generally use a treatment method that is not shown to be superior to conventional wound therapy for several different types of wounds.
Economic aspects
The cost of treating wounds with vacuum assisted closure therapy is comparable to the cost of conventional wound treatment. Hence, the method is cost-effective in treating categories of wounds for which the evidence indicates a shortened length of stay and reduced mortality. Regarding other wound categories, further clinical studies are required to show whether or not vacuum assisted wound closure therapy is cost-effective.
Four levels are used in grading the strength of the scientific evidence on which conclusions are based:
High quality evidence (![]() ). Based on high or moderate quality studies with no factors that weaken the overall assessment.
). Based on high or moderate quality studies with no factors that weaken the overall assessment.
Moderate quality evidence (![]() ). Based on high or moderate quality studies with isolated factors that weaken the overall assessment.
). Based on high or moderate quality studies with isolated factors that weaken the overall assessment.
Low quality evidence (![]() ). Based on high or moderate quality studies having factors that weaken the overall assessment.
). Based on high or moderate quality studies having factors that weaken the overall assessment.
Very low quality evidence (![]() ). Scientific evidence is deemed insufficient when scientific findings are absent, the quality of available studies is low, or studies of similar quality present conflicting findings.
). Scientific evidence is deemed insufficient when scientific findings are absent, the quality of available studies is low, or studies of similar quality present conflicting findings.
References
Included studies
- Apelqvist J, Armstrong DG, Lavery LA, Boulton AJ. Resource utilization and economic costs of care based on a randomized trial of vacuum-assisted closure therapy in the treatment of diabetic foot wounds. Am J Surg 2008;195(6):782-8.
- Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366(9498):1704-10.
- Barker DE, Green JM, Maxwell RA, Smith PW, Mejia VA, Dart BW, et al. Experience with vacuum-pack temporary abdominal wound closure in 258 trauma and general and vascular surgical patients. J Am Coll Surg 2007;204(5):784-92; discussion 92-3.
- Bee TK, Croce MA, Magnotti LJ, Zarzaur BL, Maish GO, 3rd, Minard G, et al. Temporary abdominal closure techniques: a prospective randomized trial comparing polyglactin 910 mesh and vacuum-assisted closure. J Trauma 2008;65(2):337-42; discussion 42-4.
- Bickels J, Kollender Y, Wittig JC, Cohen N, Meller I, Malawer MM. Vacuum-assisted wound closure after resection of musculoskeletal tumors. Clinical Orthopaedics and Related Research [Review] 2005;(441):346-50.
- Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006;118(2):390-7; discussion 8-400.
- Catarino PA, Chamberlain MH, Wright NC, Black E, Campbell K, Robson D, et al. High-pressure suction drainage via a polyurethane foam in the management of poststernotomy mediastinitis. Ann Thorac Surg 2000;70(6):1891-5.
- Chio EG, Agrawal A. A randomized, prospective, controlled study of forearm donor site healing when using a vacuum dressing. Otolaryngol Head Neck Surg 2010;142(2):174-8.
- Czymek R, Schmidt A, Eckmann C, Bouchard R, Wulff B, Laubert T, et al. Fournier’s gangrene: vacuum-assisted closure versus conventional dressings. Am J Surg 2009;197(2):168-76.
- Doss M, Martens S, Wood JP, Wolff JD, Baier C, Moritz A. Vacuum-assisted suction drainage versus conventional treatment in the management of poststernotomy osteomyelitis. Eur J Cardiothorac Surg 2002;22(6):934-8.
- Fuchs U, Zittermann A, Stuettgen B, Groening A, Minami K, Koerfer R. Clinical outcome of patients with deep sternal wound infection managed by vacuum-assisted closure compared to conventional therapy with open packing: a retrospective analysis. Ann Thorac Surg 2005;79(2):526-31.
- Huang WS, Hsieh SC, Hsieh CS, Schoung JY, Huang T. Use of vacuum-assisted wound closure to manage limb wounds in patients suffering from acute necrotizing fasciitis. Asian J Surg 2006;29(3):135-9.
- Kim EK, Hong JP. Efficacy of negative pressure therapy to enhance take of 1-stage allodermis and a split-thickness graft. Ann Plast Surg 2007;58(5):536-40.
- Korber A, Franckson T, Grabbe S, Dissemond J. Vacuum assisted closure device improves the take of mesh grafts in chronic leg ulcer patients. Dermatology 2008;216(3):250-6.
- Labler L, Keel M, Trentz O. Vacuum-assisted closure (V.A.C.) for temporary coverage of soft-tissue injury in type III open fracture of lower extremities. European Journal of Trauma 2004;30(5):305-12.
- Llanos S, Danilla S, Barraza C, Armijo E, Piñeros JL, Quintas M, et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg 2006;244(5):700-5.
- Moisidis E, Heath T, Boorer C, Ho K, Deva AK. A prospective, blinded, randomized, controlled clinical trial of topical negative pressure use in skin grafting. Plast Reconstr Surg 2004;114(4):917-22.
- Moues CM, van den Bemd GJ, Meerding WJ, Hovius SE. An economic evaluation of the use of TNP on full-thickness wounds. J Wound Care 2005;14(5):224-7.
- Ozturk E, Ozguc H, Yilmazlar T. The use of vacuum assisted closure therapy in the management of Fournier’s gangrene. Am J Surg 2009;197(5):660-5; discussion 5.
- Petzina R, Hoffmann J, Navasardyan A, Malmsjo M, Stamm C, Unbehaun A, et al. Negative pressure wound therapy for post-sternotomy mediastinitis reduces mortality rate and sternal re-infection rate compared to conventional treatment. European Journal of Cardio-thoracic Surgery 2010;38(1):110-3.
- Rinker B, Amspacher JC, Wilson PC, Vasconez HC. Subatmospheric pressure dressing as a bridge to free tissue transfer in the treatment of open tibia fractures. Plast Reconstr Surg 2008;121(5):1664-73.
- Scherer LA, Shiver S, Chang M, Meredith JW, Owings JT. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch Surg 2002;137(8):930-3; discussion 3-4.
- Sepúlveda G, Espíndola M, Maureira M, Sepúlveda E, Ignacio Fernández J, Oliva C, et al. [Negative-pressure wound therapy versus standard wound dressing in the treatment of diabetic foot amputation. A randomised controlled trial]. Cir Esp 2009;86(3):171-7.
- Simek M, Hajek R, Fluger I, Zalesak B, Molitor M, Lonsky V, et al. Topical negative pressure versus conventional treatment of deep sternal wound infection in cardiac surgery. EWMA Journal 2008;8(3):17-20.
- Sjogren J, Gustafsson R, Nilsson J, Malmsjo M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum-assisted closure versus conventional treatment. Ann Thorac Surg 2005;79(6):2049-55.
- Song DH, Wu LC, Lohman RF, Gottlieb LJ, Franczyk M. Vacuum assisted closure for the treatment of sternal wounds: the bridge between debridement and definitive closure. Plast Reconstr Surg 2003;111(1):92-7.
- Stannard JP, Volgas DA, Stewart R, McGwin G, Jr., Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma 2009;23(8):552-7.
- Stone P, Prigozen J, Hofeldt M, Hass S, DeLuca J, Flaherty S. Bolster versus negative pressure wound therapy for securing split-thickness skin grafts in trauma patients. Wounds: A Compendium of Clinical Research & Practice 2004;16(7):219-23.
- Yang CC, Chang DS, Webb LX. Vacuum-assisted closure for fasciotomy wounds following compartment syndrome of the leg. J Surg Orthop Adv 2006;15(1):19-23.
- Zannis J, Angobaldo J, Marks M, DeFranzo A, David L, Molnar J, et al. Comparison of fasciotomy wound closures using traditional dressing changes and the vacuum-assisted closure device. Ann Plast Surg 2009;62(4):407-9.
HTA reports
- Sullivan N, Snyder DL, Tipton DK, Uhl S, Schoelles KM. Negative pressure wound therapy devices Rockville: Agency for Healthcare Research and Quality (AHRQ).Technology Assessment. 2009.
- Vlayen J, Camberlin C, Ramaekers D. Vacuümgeassisteerde Wondbehandeling: een Rapid Assessment. Health Technology Assessment (HTA). Brussel: Federaal Kenniscentrum voor de Gezondheidszorg (KCE); 2007. KCE reports 61A (D2007/10.273/30).
Excluded studies
- Baharestani MM, Houliston-Otto DB, Barnes S. Early versus late initiation of negative pressure wound therapy: examining the impact on home care length of stay. Ostomy Wound Manage 2008;54(11):48-53.
- de Leon JM, Barnes S, Nagel M, Fudge M, Lucius A, Garcia B. Cost-effectiveness of negative pressure wound therapy for postsurgical patients in long-term acute care. Adv Skin Wound Care 2009;22(3):122-7.
- Denzinger S, Lubke L, Roessler W, Wieland WF, Kessler S, Burger M. Vacuum-assisted closure versus conventional wound care in the treatment of wound failures following inguinal lymphadenectomy for penile cancer: a retrospective study. Eur Urol 2007;51(5):1320-5.
- Gabriel A, Shores J, Heinrich C, Baqai W, Kalina S, Sogioka N, et al. Negative pressure wound therapy with instillation: A pilot study describing a new method for treating infected wounds. International Wound Journal 2008;5(3):399-413.
- Immer FF, Durrer M, Muhlemann KS, Erni D, Gahl B, Carrel TP. Deep sternal wound infection after cardiac surgery: modality of treatment and outcome. Ann Thorac Surg 2005;80(3):957-61.
- Kaplan M, Daly D, Stemkowski S. Early intervention of negative pressure wound therapy using Vacuum-Assisted Closure in trauma patients: impact on hospital length of stay and cost. Adv Skin Wound Care 2009;22(3):128-32.
- Keskin M, Karabekmez FE, Yilmaz E, Tosun Z, Savaci N. Vacuum-assisted closure of wounds and anxiety. Scand J Plast Reconstr Surg Hand Surg 2008;42(4):202-5.
- Kimball EJ, Adams DM, Kinikini DV, Mone MC, Alder SC. Delayed abdominal closure in the management of ruptured abdominal aortic aneurysm. Vascular 2009;17(6):309-15.
- Labler L, Rancan M, Mica L, Harter L, Mihic-Probst D, Keel M. Vacuum-assisted closure therapy increases local interleukin-8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma 2009;66(3):749-57.
- Lavery LA, Barnes SA, Keith MS, Seaman Jr JW, Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care 2008;31(1):26-9.
- McCallon SK, Knight CA, Valiulus JP, Cunningham MW, McCulloch JM, Farinas LP. Vacuum-assisted closure versus saline-moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy Wound Manage 2000;46(8):28-32, 34.
- Mody GN, Nirmal IA, Duraisamy S, Perakath B. A blinded, prospective, randomized controlled trial of topical negative pressure wound closure in India. Ostomy Wound Manage 2008;54(12):36-46.
- Moues CM, van den Bemd GJ, Heule F, Hovius SE. Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg 2007;60(6):672-81.
- Palmen M, van Breugel HNAM, Geskes GG, van Belle A, Swennen JMH, Drijkoningen AHM, et al. Open Window Thoracostomy Treatment of Empyema Is Accelerated by Vacuum-Assisted Closure. Annals of Thoracic Surgery 2009;88(4):1131-6.
- Perez D, Bramkamp M, Exe C, von Ruden C, Ziegler A. Modern wound care for the poor: a randomized clinical trial comparing the vacuum system with conventional saline-soaked gauze dressings. Am J Surg 2010;199(1):14-20.
- Sjogren J, Nilsson J, Gustafsson R, Malmsjo M, Ingemansson R. The impact of vacuum-assisted closure on long-term survival after post-sternotomy mediastinitis. Ann Thorac Surg 2005;80(4):1270-5.
- Stannard JP, Robinson JT, Anderson ER, McGwin G, Jr., Volgas DA, Alonso JE. Negative pressure wound therapy to treat hematomas and surgical incisions following high-energy trauma. J Trauma 2006;60(6):1301-6.
- Tauro LF, Ravikrishnan J, Satish Rao BS, Shenoy HD, Shetty SR, Menezes LT. A comparative study of the efficacy of topical negative pressure moist dressings and conventional moist dressings in chronic wounds. Indian J Plast Surg 2007;40:133-40.
- Timmers MS, Graafland N, Bernards AT, Nelissen RG, van Dissel JT, Jukema GN. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen 2009;17(2):278-86.
- Trueman P, Flack S, Loonstra A, Hauser T. The feasibility of using V.A.C. Therapy in home care patients with surgical and traumatic wounds in the Netherlands. Int Wound J 2008;5(2):225-31.
- Warner M, Henderson C, Kadrmas W, Mitchell DT. Comparison of vacuum-assisted closure to the antibiotic bead pouch for the treatment of blast injury of the extremity. Orthopedics 2010;33(2):77-82.
- Vertrees A, Greer L, Pickett C, Nelson J, Wakefield M, Stojadinovic A, et al. Modern management of complex open abdominal wounds of war: a 5-year experience. J Am Coll Surg 2008;207(6):801-9.
Other references
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4.
- GRADE Working Group. List of GRADE working group publications and grants [Internet]. [Place unknown]: GRADE Working Group, c2005-2009 [cited 2010 Mar 9].
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097.

 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services
 Share on Facebook
Share on Facebook
 Share on LinkedIn
Share on LinkedIn
 Share via Email
Share via Email