This publication was published more than 5 years ago. The state of knowledge may have changed.
Transient elastography with suspected fibrosis and cirrhosis of the liver
Summary and conclusions
Hepatitis C is the most common cause of development of fibrosis in the liver. Other causes are hepatitis B and alcohol abuse. This report has studied the scientific evidence for transient elastography to examine the degree of fibrosis development in the liver.
Conclusions
- Examining the liver using transient elastography is simpler, less risky and less painful than taking a tissue sample: liver biopsy. However the method is not equally sensitive or specific, and therefore leads to somewhat more frequent false alarms and missed cases than tissue sampling. This applies to liver disease caused by hepatitis C, hepatitis B and fatty degeneration of the liver due to alcohol. No definite conclusions can be drawn regarding the applicability of the method with non-alcohol-related fatty degeneration of the liver.
- If we disregard that the method is not as sensitive or specific, annual examinations using transient elastography appear to be a cheaper and less painful alternative to a liver biopsy performed every three years. To be able to calculate the method’s cost effectiveness, the costs of the examination must be considered in relation to how the accuracy of the method affects survival, quality of life and future health care costs.
Patient benefit/evidence-graded results
- With hepatitis C, there is low quality of evidence (
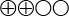 ) that transient elastography displays 81 percent sensitivity and 83 percent specificity for stage 2 fibrosis, and 86 percent sensitivity and 89 percent specificity for stage 4 fibrosis.
) that transient elastography displays 81 percent sensitivity and 83 percent specificity for stage 2 fibrosis, and 86 percent sensitivity and 89 percent specificity for stage 4 fibrosis. - With hepatitis B, there is low quality of evidence (
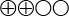 ) that transient elastography displays 80 percent sensitivity and 86 percent specificity for stage 2 fibrosis, and 60 percent sensitivity and 81 percent specificity for stage 4 fibrosis.
) that transient elastography displays 80 percent sensitivity and 86 percent specificity for stage 2 fibrosis, and 60 percent sensitivity and 81 percent specificity for stage 4 fibrosis. - There is very low quality of evidence (
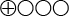 ) regarding the diagnostic reliability of transient elastography with non-alcohol-related fatty degeneration of the liver.
) regarding the diagnostic reliability of transient elastography with non-alcohol-related fatty degeneration of the liver. - With alcohol-related fatty degeneration of the liver, there is low quality of evidence (
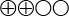 ) that transient elastography displays 84 percent sensitivity and moderate quality of evidence (
) that transient elastography displays 84 percent sensitivity and moderate quality of evidence (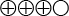 ) of 90 percent specificity for stage 2 fibrosis, and moderate quality of evidence (
) of 90 percent specificity for stage 2 fibrosis, and moderate quality of evidence (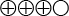 ) of 85 percent sensitivity and 84 percent specificity for stage 4 fibrosis.
) of 85 percent sensitivity and 84 percent specificity for stage 4 fibrosis. - With mixed etiology (several causes), there is low quality of evidence (
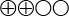 ) that transient elastography displays 78 percent sensitivity and 84 percent specificity for stage 2 fibrosis, and 74 percent sensitivity and 88 percent specificity for stage 4 fibrosis.
) that transient elastography displays 78 percent sensitivity and 84 percent specificity for stage 2 fibrosis, and 74 percent sensitivity and 88 percent specificity for stage 4 fibrosis.
For more on this report, please download the full text report
Tables in English: Table 1a Characteristics and quality assessment of included systematic reviews on transient elastography, page 8; Table 1b Characteristics and quality assessment of included original studies on transient elastography, page 8; Table 2 Outcome results of included studies on transient elastography, page 13
References, page 21
The quality of evidence is graded into four levels according to the GRADE system:
High quality of evidence (![]() ). Based on high or medium quality studies without weakening factors in an overall assessment.
). Based on high or medium quality studies without weakening factors in an overall assessment.
Moderate quality of evidence (![]() ). Based on high or medium quality studies with the existence of occasional weakening factors in an overall assessment.
). Based on high or medium quality studies with the existence of occasional weakening factors in an overall assessment.
Low quality of evidence (![]() ). Based on high or medium quality studies with weakening factors in an overall assessment.
). Based on high or medium quality studies with weakening factors in an overall assessment.
Very low quality of evidence (![]() ). When scientific evidence is lacking, where the available studies are of low quality or where studies of similar quality show contradictory results, the scientific evidence is stated to be inadequate.
). When scientific evidence is lacking, where the available studies are of low quality or where studies of similar quality show contradictory results, the scientific evidence is stated to be inadequate.
The grading of evidence according to GRADE is in the first instance based on high or medium quality studies, where such exist. If there are both randomised controlled trials (RCTs) and observational studies of the same quality, the grading of evidence is based on RCTs.
How to cite this report: SBU. Transient elastography with suspected fibrosis and cirrhosis of the liver. Stockholm: Swedish Council on Health Technology Assessment (SBU); 2013. SBU Alert report no 2013 01. ISSN 1652-7151. http://www.sbu.se/201301e

 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services
 Share on Facebook
Share on Facebook
 Share on LinkedIn
Share on LinkedIn
 Share via Email
Share via Email