This publication was published more than 5 years ago. The state of knowledge may have changed.
Blood Test for Early Diagnosis of Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common cause of dementia [1]. The scientific literature offers a good description of the tissue changes in the brain resulting from Alzheimer’s disease. The prevalence of certain substances, biomarkers, in the cerebrospinal fluid reflects these changes. Alzheimer’s disease can be diagnosed by testing cerebrospinal fluid obtained by lumbar puncture (spinal tap). However, a blood test would be an easier way to diagnose the disease.
Four potential biomarkers that can be measured in plasma and serum have been studied for Alzheimer’s disease: plasma or serum levels of amyloid β (Aβ), autoantibodies against Aβ, platelet amyloid precursor protein (platelet APP), and α1 antichymotrypsin (ACT).
Conclusions
- Currently, no biomarkers in blood can be used to diagnose Alzheimer’s disease.
- Of the four biomarkers studied, only platelet APP has shown differences between sick and healthy individuals. For the other biomarkers, the difference between sick and healthy people is insignificant.
- Large, independent studies are needed to determine whether platelet APP in blood tests could serve as a diagnostic tool.
- Studies using refined, highly sensitive measurement methods are needed to identify more biomarkers that could serve as diagnostic tools.
Method and target group
Alzheimer’s disease mainly affects the elderly, but the early-onset AD can debut before 65 years of age [1]. Most cases of AD are sporadic, while genetically inherited forms comprise less than 0.1% of cases. Inheritance is autosomal dominant. Today, patients with mild memory loss often seek care. At this early stage of the disease, diagnostic biomarkers that reveal the underlying disease process would be valuable. Such biomarkers are found in the cerebrospinal fluid (total tau, phosphorylated tau, and the 42-amino-acid-long isoform of amyloid β [Aβ42]). The biomarkers can be used to diagnose Alzheimer’s disease with a sensitivity and specificity of 80% to 95%, 5 to 10 years before the patient meets the criteria for dementia [2]. Nevertheless, it would be desirable to have markers in blood that could be used diagnostically to avoid spinal tap, which is a more difficult and time consuming procedure than blood testing.
This report covers the literature on biomarkers in blood for diagnosis of Alzheimer’s disease. We have included case-control studies and longitudinal studies of patients with mild cognitive disorders that later develop into Alzheimer’s disease (prodromal Alzheimer’s) [3].
Question
- What is the diagnostic accuracy of plasma or serum levels of Aβ, autoantibodies against Aβ, platelet APP ratio, and ACT in distinguishing people with Alzheimer’s disease from cognitively healthy controls?
Patient benefits
- The scientific evidence is insufficient (two studies from the same research group and with deficiencies regarding study quality and directness) to determine whether the platelet APP ratio in blood tests can be used to estimate diagnostic accuracy in identifying Alzheimer’s disease (
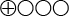 ).
).
This assessment included in total 45 studies: 21 on plasma or serum levels of Aβ, 8 on autoantibodies against Aβ, 6 on platelet APP ratio, and 10 on ACT. Study populations consisted mainly of patients with Alzheimer’s disease who were compared with cognitively healthy controls. Some studies also investigated the association between the markers and future Alzheimer’s disease in longitudinal cohorts of cognitively healthy elderly, or patients with mild cognitive symptoms that did not meet the criteria for Alzheimer’s disease when tested. All studies used the clinical NINCDS-ADRDA criteria from 1984 as a standard reference [4]. Some studies also used DSM or ICD criteria.
Most of the studies found no, or clinically insignificant, differences between Alzheimer’s patients and controls for the biomarkers studied. The studies on Aβ, autoantibodies against Aβ, and ACT were excluded at this stage (39 studies) since they did not show any diagnostically relevant differences.
Six studies addressing platelet APP ratios show potentially useful diagnostic differences between clinically relevant comparison groups. Four of the six studies were excluded since they did not report on diagnostic accuracy. The two remaining studies were found to have medium quality, but were produced by the same research group. Hence, large and independent studies are needed.
Ethical aspects
If, in the future, a clinically useful blood test becomes available to diagnose the disease – in the absence of effective treatment – ethical considerations would be necessary. The possibility for early diagnosis, but not treatment, of Alzheimer’s disease would cause distress for patients and families. In the worst case, a diagnosis could stigmatise people in a very early stage of Alzheimer’s disease, even though they might never develop a serious case of the disease.
A clinically useful blood test could have several positive effects. For instance, early diagnosis could help explain altered and perhaps unusual behaviour. An early diagnosis could also increase opportunities to take steps in preparing for the later phase of disease.
Economic aspects
Costs and cost effectiveness were not analysed since accurate and diagnostically useful methods have yet to be identified.
Four levels are used in grading the strength of the scientific evidence on which conclusions are based:
Strong scientific evidence (![]() ). Based on high or medium quality studies with no factors that weaken the overall assessment.
). Based on high or medium quality studies with no factors that weaken the overall assessment.
Moderately strong scientific evidence (![]() ). Based on high or medium quality studies with isolated factors that weaken the overall assessment.
). Based on high or medium quality studies with isolated factors that weaken the overall assessment.
Limited scientific evidence (![]() ). Based on high or medium quality studies having factors that weaken the overall assessment.
). Based on high or medium quality studies having factors that weaken the overall assessment.
Insufficient scientific evidence (![]() ). Scientific evidence is deemed insufficient when scientific findings are absent, the quality of available studies is low, or studies of similar quality present conflicting findings.
). Scientific evidence is deemed insufficient when scientific findings are absent, the quality of available studies is low, or studies of similar quality present conflicting findings.
References
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet 2006;368:387-403.
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010;6:131-44.
- Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 2010;9:1118-27.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939-44.
- McConnell LM, Sanders GD, Owens DK. Evaluation of genetic tests: APOE genotyping for the diagnosis of Alzheimer disease. Genet Test 1999;3:47-53.
- Blasko I, Jellinger K, Kemmler G, Krampla W, Jungwirth S, Wichart I, et al. Conversion from cognitive health to mild cognitive impairment and Alzheimer’s disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiol Aging 2008;29:1-11.
- Padovani A, Borroni B, Colciaghi F, Pettenati C, Cottini E, Agosti C, et al. Abnormalities in the pattern of platelet amyloid precursor protein forms in patients with mild cognitive impairment and Alzheimer disease. Arch Neurol 2002;59:71-5.
- Padovani A, Pastorino L, Borroni B, Colciaghi F, Rozzini L, Monastero R, et al. Amyloid precursor protein in platelets: a peripheral marker for the diagnosis of sporadic AD. Neurology 2001;57:2243-8.
- Lieberman J, Schleissner L, Tachiki KH, Kling AS. Serum alpha 1-antichymotrypsin level as a marker for Alzheimer-type dementia. Neurobiol Aging 1995;16:747-53.
- Baranowska-Bik A, Bik W, Wolinska-Witort E, Martynska L, Chmielowska M, Barcikowska M, et al. Plasma beta amyloid and cytokine profile in women with Alzheimer’s disease. Neuro Endocrinol Lett 2008;29:75-9.
- Brettschneider S, Morgenthaler NG, Teipel SJ, Fischer-Schulz C, Burger K, Dodel R, et al. Decreased serum amyloid beta(1-42) autoantibody levels in Alzheimer’s disease, determined by a newly developed immuno-precipitation assay with radiolabeled amyloid beta(1-42) peptide. Biol Psychiatry 2005;57:813-6.
- Xu W, Kawarabayashi T, Matsubara E, Deguchi K, Murakami T, Harigaya Y, et al. Plasma antibodies to Abeta40 and Abeta42 in patients with Alzheimer’s disease and normal controls. Brain Res 2008;1219:169-79.
- Licastro F, Masliah E, Pedrini S, Thal LJ. Blood levels of alpha-1-antichymotrypsin and risk factors for Alzheimer’s disease: effects of gender and apolipoprotein E genotype. Dement Geriatr Cogn Disord 2000;11:25-8.
- Matsubara E, Ghiso J, Frangione B, Amari M, Tomidokoro Y, Ikeda Y, et al. Lipoprotein-free amyloidogenic peptides in plasma are elevated in patients with sporadic Alzheimer’s disease and Down’s syndrome. Ann Neurol 1999;45:537-41.
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol 2004;61:668-72.
- Thambisetty M, Simmons A, Velayudhan L, Hye A, Campbell J, Zhang Y, et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry 2010;67:739-48.
- Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med 2007;13:1359-62.
- Hye A, Lynham S, Thambisetty M, Causevic M, Campbell J, Byers HL, et al. Proteome-based plasma biomarkers for Alzheimer’s disease. Brain 2006;129:3042-50.
- Tamaoka A, Fukushima T, Sawamura N, Ishikawa K, Oguni E, Komatsuzaki Y, et al. Amyloid beta protein in plasma from patients with sporadic Alzheimer’s disease. J Neurol Sci 1996;141:65-8.
- Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol 2000;57:100-5.
- Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, et al. Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology 2003;61:1185-90.
- Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol 2003;60:958-64.
- Sobow T, Flirski M, Kloszewska I, Liberski PP. Plasma levels of alpha beta peptides are altered in amnestic mild cognitive impairment but not in sporadic Alzheimer’s disease. Acta Neurobiol Exp (Wars) 2005;65:117-24.
- van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Abeta(1-40) and Abeta(1-42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol 2006;5:655-60.
- Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol 2007;64:354-62.
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 2007;64:343-9.
- Abdullah L, Paris D, Luis C, Quadros A, Parrish J, Valdes L, et al. The influence of diagnosis, intra- and inter-person variability on serum and plasma Abeta levels. Neurosci Lett 2007;428:53-8.
- Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA, et al. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology 2008;70:1664-71.
- Schupf N, Tang MX, Fukuyama H, Manly J, Andrews H, Mehta P, et al. Peripheral Abeta subspecies as risk biomarkers of Alzheimer’s disease. Proc Natl Acad Sci U S A 2008;105:14052-7.
- Ait-ghezala G, Abdullah L, Volmar CH, Paris D, Luis CA, Quadros A, et al. Diagnostic utility of APOE, soluble CD40, CD40L, and Abeta1-40 levels in plasma in Alzheimer’s disease. Cytokine 2008;44:283-7.
- Frankfort SV, van Campen JP, Tulner LR, Beijnen JH. Serum amyloid beta peptides in patients with dementia and age-matched non-demented controls as detected by surface-enhanced laser desorption ionisation-time of flight mass spectrometry (SELDI-TOF MS). Curr Clin Pharmacol 2008;3:144-54.
- Sundelof J, Giedraitis V, Irizarry MC, Sundstrom J, Ingelsson E, Ronnemaa E, et al. Plasma beta amyloid and the risk of Alzheimer disease and dementia in elderly men: a prospective, population-based cohort study. Arch Neurol 2008;65:256-63.
- Lambert JC, Schraen-Maschke S, Richard F, Fievet N, Rouaud O, Berr C, et al. Association of plasma amyloid beta with risk of dementia: the prospective Three-City Study. Neurology 2009;73:847-53.
- Luis CA, Abdullah L, Paris D, Quadros A, Mullan M, Mouzon B, et al. Serum beta-amyloid correlates with neuropsychological impairment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2009;16:203-18.
- Le Bastard N, Aerts L, Leurs J, Blomme W, De Deyn PP, Engelborghs S. No correlation between time-linked plasma and CSF Abeta levels. Neurochem Int 2009;55:820-5.
- Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging 2010;31:357-67.
- Weksler ME, Relkin N, Turkenich R, LaRusse S, Zhou L, Szabo P. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp Gerontol 2002;37:943-8.
- Mruthinti S, Buccafusco JJ, Hill WD, Waller JL, Jackson TW, Zamrini EY, et al. Autoimmunity in Alzheimer’s disease: increased levels of circulating IgGs binding Abeta and RAGE peptides. Neurobiol Aging 2004;25:1023-32.
- Moir RD, Tseitlin KA, Soscia S, Hyman BT, Irizarry MC, Tanzi RE. Autoantibodies to redox-modified oligomeric Abeta are attenuated in the plasma of Alzheimer’s disease patients. J Biol Chem 2005;280:17458-63.
- Jianping L, Zhibing Y, Wei Q, Zhikai C, Jie X, Jinbiao L. Low avidity and level of serum anti-Abeta antibodies in Alzheimer disease. Alzheimer Dis Assoc Disord 2006;20:127-32.
- Song MS, Mook-Jung I, Lee HJ, Min JY, Park MH. Serum anti-amyloid-beta antibodies and Alzheimer’s disease in elderly Korean patients. J Int Med Res 2007;35:301-6.
- Gustaw KA, Garrett MR, Lee HG, Castellani RJ, Zagorski MG, Prakasam A, et al. Antigen-antibody dissociation in Alzheimer disease: a novel approach to diagnosis. J Neurochem 2008;106:1350-6.
- Di Luca M, Pastorino L, Bianchetti A, Perez J, Vignolo LA, Lenzi GL, et al. Differential level of platelet amyloid beta precursor protein isoforms: an early marker for Alzheimer disease. Arch Neurol 1998;55:1195-200.
- Borroni B, Colciaghi F, Corsini P, Akkawi N, Rozzini L, Del Zotto E, et al. Early stages of probable Alzheimer disease are associated with changes in platelet amyloid precursor protein forms. Neurol Sci 2002;23:207-10.
- Sanchez-Gonzalez VJ, Ortiz GG, Gallegos-Arreola P, Macias-Islas MA, Arias-Merino ED, Loera-Castaneda V, et al. Altered beta-amyloid precursor protein isoforms in Mexican Alzheimer’s Disease patients. Dis Markers 2006;22:119-25.
- Zainaghi IA, Forlenza OV, Gattaz WF. Abnormal APP processing in platelets of patients with Alzheimer’s disease: correlations with membrane fluidity and cognitive decline. Psychopharmacology (Berl) 2007;192:547-53.
- Pirttila T, Mehta PD, Frey H, Wisniewski HM. Alpha 1-antichymotrypsin and IL-1 beta are not increased in CSF or serum in Alzheimer’s disease. Neurobiol Aging 1994;15:313-7.
- Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, et al. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer’s disease: peripheral inflammation or signals from the brain? J Neuroimmunol 2000;103:97-102.
- McIlroy SP, Vahidassr MD, Savage DA, Lloyd F, Patterson CC, Lawson JT, et al. Association of serum AACT levels and AACT signal polymorphism with late-onset Alzheimer’s disease in Northern Ireland. Int J Geriatr Psychiatry 2000;15:260-6.
- DeKosky ST, Ikonomovic MD, Wang X, Farlow M, Wisniewski S, Lopez OL, et al. Plasma and cerebrospinal fluid alpha1-antichymotrypsin levels in Alzheimer’s disease: correlation with cognitive impairment. Ann Neurol 2003;53:81-90.
- Nielsen HM, Minthon L, Londos E, Blennow K, Miranda E, Perez J, et al. Plasma and CSF serpins in Alzheimer disease and dementia with Lewy bodies. Neurology 2007;69:1569-79.
- Porcellini E, Davis EJ, Chiappelli M, Ianni E, Di Stefano G, Forti P, et al. Elevated plasma levels of alpha-1-anti-chymotrypsin in age-related cognitive decline and Alzheimer’s disease: a potential therapeutic target. Curr Pharm Des 2008;14:2659-64.

 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services
 Share on Facebook
Share on Facebook
 Share on LinkedIn
Share on LinkedIn
 Share via Email
Share via Email