Postcovid – behandling och rehabilitering
En evidenskarta – juni 2022
Sammanfattning
SBU fick i uppdrag att löpande utvärdera och sprida kunskap om det vetenskapliga stödet avseende behandling och rehabilitering av patienter med långvariga effekter av sjukdomen covid-19 (S2021/02146 (delvis)). Slutrapportering skulle ske senast den 15 augusti år 2022.
För att göra information lätt tillgänglig för en allmänhet utvecklades en evidenskarta som publicerades den 26 augusti år 2021. Se www.sbu.se/328#karta. Där hänvisas även till andra aktörer som arbetar med frågor om covid-19.
Sammanställningen av den vetenskapliga litteraturen har genomförts via litteratursökning i internationella databaser, relevansbedömning och granskning av risk för systematiska fel (bias) i enskilda studier. Avslutningsvis gjordes en bedömning av resultatens tillförlitlighet.
Fram till den 1 juni år 2022, när den sista litteratursökningen genomfördes, hade 19 studier identifierats om behandling och rehabilitering vid postcovid (kortform för diagnoskoden i Patientregistret). Av dessa bedömdes åtta studier ha hög risk för systematiska fel i resultaten. De resterande elva studierna, som bedömts vara tillräckligt välgjorda, beskrivs kort i denna slutredovisning av uppdraget. Resultaten är uppdelade enligt de vanligaste symtom eller besvär vid postcovid som rapporterats in till Patientregistret från vården.
Dessa elva studier har undersökt behandling eller rehabilitering vid nedsatt lungfunktion eller andning, hjärntrötthet eller kognitiv nedsättning, smärta, posttraumatisk stress, lukt- eller smakbortfall samt allmän trötthet. Studierna var gjorda i Kina, Italien, Belgien, Storbritannien och Turkiet. Totalt deltog 1 175 patienter, som antingen vårdats på sjukhus eller fått någon form av öppenvård. Könsfördelning varierade i de olika studierna (från 19 % till 70 % män) och medelåldern varierade mellan 42 och 59 år.
Sammantaget har det vetenskapliga underlaget mycket låg tillförlitlighet. Det går därmed inte att bedöma om någon av de studerade behandlingarna eller rehabiliterande åtgärderna är effektiva eller inte, utifrån det underlag som SBU har identifierat. Det betyder inte att behandlingarna inte har någon effekt, utan att det behövs fler välgjorda studier för att kunna bedöma effekten.
I uppdraget ingick att särskilt uppmärksamma effekter av covid-19 på barn, men ingen studie har identifierats som handlar om behandling för barn med långvariga symtom efter covid-19.
Under uppdragstiden har SBU löpande redovisat resultat till Socialstyrelsen, Vetenskapsrådet och Myndigheten för vård- och omsorgsanalys, samt kontinuerligt på SBU:s webbsida.
Som en bakgrund till resultatet i denna rapport presenteras några uppgifter från Patientregistret om förekomsten av postcovid. Den 7 juni år 2022 redovisade Socialstyrelsen att totalt 8 221 personer hade fått vård för postcovid, främst inom slutenvården eller av läkare inom den öppna specialistvården i Sverige.
Med tanke på den pågående forskningsaktiviteten är det rimligt att förvänta sig avsevärt mer kunskap om covid-19 och långvariga effekter av infektionen under de kommande åren.
1. Inledning
1.1 Uppdrag
SBU fick den 4 mars år 2021 ett regeringsuppdrag om att löpande utvärdera och sprida kunskap om det vetenskapliga stödet avseende långvariga effekter av sjukdomen covid-19 (S2021/02146 (delvis)).
Enligt uppdraget skulle SBU:
- Utveckla en metod för att kontinuerligt publicera det vetenskapliga underlaget avseende behandling och rehabilitering av patienter med långvariga effekter av sjukdomen covid-19.
- Presentera resultatet på ett översiktligt, tillgängligt och visuellt sätt.
- Särskilt uppmärksamma effekter av covid-19 på barn.
- I samverkan med Socialstyrelsen ta fram ett arbetssätt så att resultat och bedömningar löpande kunde förmedlas till Socialstyrelsen.
- Följa och utvärdera andra internationella aktörers arbete med frågan.
- Utveckla ett arbetssätt för att löpande identifiera områden där det finns behov av forskning avseende behandling och rehabilitering av långvariga effekter av covid-19.
SBU skulle också kommunicera resultat och forskningsbehov till Vetenskapsrådet samt andra relevanta myndigheter och aktörer. Om statistik sammanställs ska kön och ålder beaktas. Frågorna för det vetenskapliga underlaget ska tas fram i nära dialog med Socialstyrelsen.
Uppdraget har delredovisats den 31 maj år 2021, den 28 februari och den 31 maj år 2022 till Regeringskansliet. Detta är slutrapporteringen som ska överlämnas senast den 15 augusti år 2022 till Regeringskansliet.
Syftet med uppdraget är främst att öka kunskapen om behandling och rehabilitering av patienter med långvariga symtom efter sjukdomen covid-19. Postcovid1 är en term för efterföljande tillstånd utan infektion, vilka kan särskiljas från en pågående infektion med covid-19 [1] [2]. Tillståndet kallas även postakut covid-19-syndrom [3]. Syftet är vidare att löpande sprida kunskapen om det vetenskapliga stödet på ett översiktligt, tillgängligt och visuellt sätt.
1. Postcovid = Kortform för tilläggskoden Postinfektiöst tillstånd efter covid-19, ospecificerat (ICD-10 U0.9.9). Koden infördes i Sverige den 16 oktober år 2020, enligt ett tidigare beslut från World Health Organization (WHO). Tilläggskoden bör användas efter den kod som beskriver det aktuella kvarstående symtomet eller sena besväret (till exempel huvuddiagnos Anosmi, R43.0, tilläggskod U09.9).
1.2 Antal personer med postcovid
Socialstyrelsen publicerar månadsvis statistik om covid-19, bland annat om förekomsten av postcovid [4]. Den 7 juni år 2022 redovisades att totalt 8 221 personer hade fått vård för postcovid, främst inom slutenvården eller av läkare inom den öppna specialistvården i Sverige. Av dessa var ungefär hälften kvinnor (cirka 53 %), en majoritet var personer i arbetsför ålder (cirka 77 %), medan färre var äldre (cirka 18 %) eller barn (cirka 5 %) (Figur 1.1).
Dessa uppgifter bör enligt Socialstyrelsen betraktas som preliminära, eftersom en del av underlaget kommer från frivillig regional rapportering samt att uppgifter från primärvården saknas [4]. Vidare kan felkällor finnas från registreringen av denna nya diagnoskod.
Socialstyrelsen redovisade även uppgifter om det totala antalet unika personer som fått vård för covid-19, vilket var 30 031 personer. Av dessa hade 19 414 personer vårdats på sjukhus, varav 619 personer vårdats på intensivvårdsavdelning. Uppgifterna gällde fram till 6 juni år 2022.
1.3 Vanligaste symtomen vid postcovid
Socialstyrelsen publicerar även uppgifter från Patientregistret om vilka symtom eller besvär (diagnoskoder enligt ICD-10) som främst har rapporterats tillsammans med tilläggsdiagnosen postcovid [4]. Uppgifter till Patientregistret ska lämnas av vårdgivare om patienter som vårdas eller har vårdats på sjukhus, eller av läkare i öppenvård som inte är att betrakta som primärvård. Av Tabell 1.1 framgår det att de vanligaste symtom som rapporterats var besvär med lungfunktion/andning och hjärntrötthet/kognitiv nedsättning. Symtom hos patienter som genomgått covid-19 och som behandlats inom primärvården (husläkare, hälsocentral och liknande) ingår inte i statistiken från Patientregistret.
| * Mer än ett besvär (diagnos) kan registreras per person, därför blir summan av andelarna mer än 100 procent. Om en person har samma besvär vid flera vårdtillfällen/läkarbesök räknas diagnosen bara en gång. | ||
| Diagnosgrupper (ICD-10-SE-koder) | Antal personer | Andel av totalt antal personer* |
| Lungfunktion/Andning | 3 350 | 41 % |
| Hjärntrötthet/Kognitiv funktionsnedsättning | 2 546 | 31 % |
| Smärta | 1 236 | 15 % |
| Hjärtklappning/POTS | 855 | 10 % |
| Kol/Astma | 596 | 7 % |
| Pneumoni | 455 | 6 % |
| Njurbesvär | 354 | 4 % |
| Lukt/Smak | 302 | 4 % |
| Neurologiska besvär | 275 | 3 % |
| Sömnproblem | 263 | 3 % |
| Feber | 243 | 3 % |
| Yrsel/Illamående | 242 | 3 % |
| Depression/Ångest | 228 | 3 % |
1.4 Antal personer med multisystemiskt inflammatoriskt syndrom
Socialstyrelsen publicerar även uppgifter om antalet patienter med covid-19 och multisystemiskt inflammatoriskt syndrom, som är ett ovanligt men allvarligt hyperinflammatoriskt tillstånd. Det är inte ett strikt postcovid-syndrom, då det även kan förekomma under pågående covid-19. Därför klassificeras den diagnosen som en egen kategori.
Totalt hade 710 personer diagnostiserats med både covid-19 och multisystemiskt inflammatoriskt syndrom fram till 7 juni år 2022. Av Figur 1.2 framgår det att flertalet är barn (53 %).
2. Metod
En dialog fördes initialt med Socialstyrelsen om vilken eller vilka frågor som man kunde besvara i detta regeringsuppdrag. Den konkreta fråga som därefter formulerades var: Vilka behandlings- och rehabiliteringsinsatser är effektiva för patienter med postcovid?
Frågan besvarades via en systematisk litteratursökning av den internationella vetenskapliga litteraturen. De relevanta artiklar som identifierades, och som bedömdes vara tillräckligt välgjorda (måttlig risk för bias), har sammanställts på ett överskådligt sätt. För att strukturera frågan inför systematiska litteratursökningar formulerades nedanstående inklusionskriterier.
2.1 Inklusionskriterier
Population:
- Personer med postcovid enligt WHO:s definition (personer som uppvisar kvarvarande eller nya symtom efter genomgången infektion minst 3 månader från insjuknande, där symtomen varat i minst 2 månader och inte kan förklaras av annan diagnos).
- Personer som inte uppnådde kriterierna enligt WHO:s definition (men hade symtom och påbörjade behandling efter genomgången infektion och följdes i minst tre månader).
Intervention:
- Behandlings- eller rehabiliteringsinsatser mot relevanta symtom eller följdsjukdom.
Jämförelseintervention:
- Ingen behandling eller annan behandling.
Utfallsmått:
- Samtliga utfall som relaterats till postcovid, exempelvis fysisk förmåga, andningsbesvär, sjukdomskänsla, livskvalitet, komplikationer av behandling eller rehabilitering och dödlighet.
Studiedesign:
- Kontrollerade studier2.
WHO publicerade i oktober år 2021 ett konsensusdokument om en klinisk definition av postcovid. Där framgår att tidsgränsen vanligen är tre månader efter sjukdomsdebut och att symtomen ska pågå i minst två månader [5]. Avgränsningen för vilka patienter som är relevanta (population) i denna rapport preciserades något vid två tillfällen i samråd med projektets sakkunniga och SBU:s vetenskapliga råd, i augusti respektive december år 2021.
2. Kontrollerade studier = Kliniska studier, med eller utan slumpmässigt urval av patienter till behandlings- och jämförelsegrupp.
2.2 Litteratursökning
En systematisk litteratursökning, i internationella databaser med fokus på behandling och rehabilitering, har genomförts som ett komplement till den sökning som gjordes i ett tidigare regeringsuppdrag av SBU om långvariga symtom vid covid-19 [6]. Från och med maj år 2021 har sökningen upprepats veckovis i en databas (Medline Ovid). Månatligen har sökningen kompletterats i fem andra databaser (Cinahl, PsycInfo, Cochrane Library, Embase och WHO COVID-19: Global literature on coronavirus disease). Dessa uppdateringar har skett kontinuerligt under uppdragstiden. Därutöver har projektgruppen bevakat de internationella covid-19-specifika resurserna Covid-NMA och Cochrane Rehabilitation samt referenslistor i relevant litteratur (se lista över relevanta översikter). Eftersom uppdraget skulle slutredovisas senast den 15 augusti år 2022 gjordes den sista litteratursökning den 1 juni år 2022. Litteratursökningen redovisas i detalj i Bilaga 1.
2.3 Urval och granskning av litteratur
De artiklar som inte bedömdes vara relevanta för frågeställningen exkluderades (se listan över exkluderade studier). De artiklar som bedömts vara relevanta enligt de inklusionskriterier som gjorts granskades närmare avseende risk för bias3 med hjälp av SBU:s granskningsmallar (Bilaga 2). För att bedöma relevansen och risken för bias granskade två personer artiklarna oberoende av varandra. Oenighet i bedömningarna löstes genom diskussion mellan granskarna och vid behov tillfrågades minst en tredje sakkunnig bedömare. De artiklar som bedömdes ha en hög risk för bias inkluderades inte i resultatet, men presenterades i den ”levande evidenskartan”. Syftet var att visa vilka vetenskapliga artiklar som ändå är relevanta för frågan.
3. Bias = Systematiskt fel. Ett resultatfel i forskningsprocessen som uppstått i en studies upplägg, genomförande, effektbedömning, publikation eller annan hantering av resultaten, och som inte beror på slumpen.
Vissa artiklar har enbart haft en engelsk sammanfattning, men resterande text på andra språk än engelska, norska, danska eller svenska. När dessa utifrån enbart sammanfattningen verkat vara relevanta för frågan för båda bedömarna, då har de listats särskilt på SBU:s webbsida (se lista över relevanta artiklar). De aktuella andra språken har varit ett fåtal kinesiska och ett flertal ryskspråkiga artiklar. För några av de ryska artiklarna har SBU fått stöd i att förstå relevansen av en översättare.
Hur det systematiska arbetet med att söka litteratur, göra urval och granska vetenskapliga artiklar genomförs illustreras i Figur 2.1.
2.4 Syntes utan metaanalys
De inkluderade studierna bedömdes vara alltför olika avseende population, intervention och utfall för att kunna slås samman. Endast en liten pilotstudie kunde slås samman med en annan studie som gjordes senare av samma forskargrupp. Dessa sammanvägdes narrativt i en syntes utan metaanalys. Resultaten består i resten av fallen av en sammanfattning av enskilda studier.
2.5 Bedömning av tillförlitlighet
En bedömning har gjorts av tillförlitligheten till resultaten i det vetenskapliga underlaget. Den strukturerade bedömningen har gjorts enligt det internationella systemet GRADE (Faktaruta 2.1 och SBU:s metodbok [7]). Samtliga avdrag är angivna i de sammanfattande tabellerna i Kapitel 4, även om den totala GRADE-bedömningen som lägst kan bli +1 (mycket låg tillförlitlighet).
2.6 Kontinuerlig publicering av resultat
En digital redovisningsform utvecklades i form av en ”levande evidenskarta”, som publicerades den 26 augusti år 2021. Se www.sbu.se/328#karta. Motsvarande information finns också på engelska, www.sbu.se/328e.
Resultat fram till den 1 juni år 2022 har uppdaterats varje vecka med information om antalet nya identifierade sökträffar i internationella databaser. Efter urval och granskning av artiklar presenteras resultatet på tre sätt:
- Så snart en artikel bedömts vara relevant, det vill säga att den handlar om behandling eller rehabilitering vid postcovid, redovisas den i evidenskartan på hemsidan. En hänvisning ges till artikeln eller sammanfattningen via en länk.
- De relevanta artiklar som bedömts vara tillräckligt välgjorda (måttlig risk för bias), sammanfattas dessutom på engelska i tabellform. Se listan över relevanta studier. Här listas även artiklar på andra språk, som av den engelska sammanfattningen verkar vara potentiellt relevanta.
- Artiklar som inte bedömts vara relevanta efter granskning i fulltext, har listats separat (se listan över exkluderade studier). På listan anges också det främsta skälet till varför en artikel inte inkluderats i resultatet. Flertalet artiklar görs tillgängliga för den intresserade läsaren via en länk.
2.7 Samverkan med andra aktörer
En dialog har initialt förts med Socialstyrelsen, om vilka frågor som kan besvaras i det här uppdraget. Därefter har ett samråd via digitala möten fortsatt, där Socialstyrelsen löpande har tagit del av det resultat som SBU fått fram. En dialog har även förts med Vetenskapsrådet, om hur SBU successivt kan informera om vilka vetenskapliga kunskapsluckor som eventuellt kommer att identifieras. Informationen har främst getts i samband med att delredovisningar lämnats till Regeringskansliet. SBU har även informerat Myndigheten för vård- och omsorgsanalys om detta uppdrag, med anledning av deras regeringsuppdrag om att kartlägga om det finns regionala skillnader i vård och omsorg av patienter med postcovid. En delrapport har publicerats av Myndigheten för vård- och omsorgsanalys i mars år 2022 [8].
Sedan den 26 augusti år 2021, när SBU publicerade en webbplats (se avsnitt Kontinuerlig publicering av resultat), har både Socialstyrelsen, Vetenskapsrådet och andra intresserade fortlöpande haft tillgång till en sammanställning av resultatet.
Med hjälp av de nationella programområdena (NPO), inom Nationellt system för kunskapsstyrning hälso- och sjukvård, har SBU fått förslag på sakkunniga personer med olika specialiteter och viss geografisk spridning. Under avsnittet Projektgrupp listas vilka personer som engagerats och från vilka NPO.
För att följa andra aktörers arbete hänvisar SBU, på sin webbsida https://www.sbu.se/328 under rubriken Lästips, till internationella webbsidor som redovisar vetenskapliga underlag som rör olika frågor om sjukdomen covid-19.
Projektet har registrerats i International prospective register of systematic reviews (https://www.crd.york.ac.uk/PROSPERO), med id-nr: CRD42021276717.
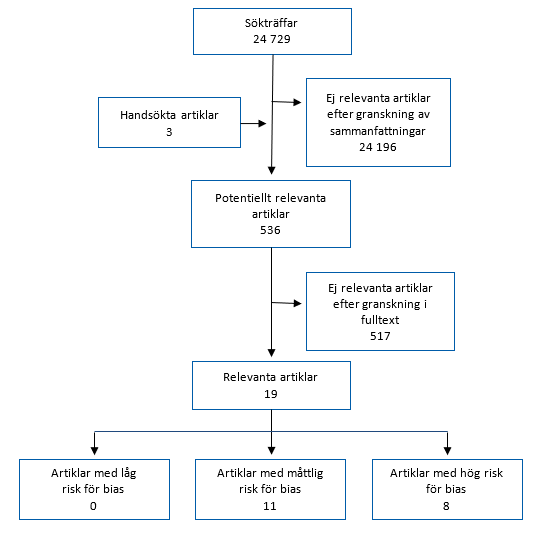
3. Urval av studier
Sedan den litteratursökning som gjordes den 2 november år 2020 [6], om långvariga symtom vid covid-19, har SBU fram till den 1 juni år 2022 gått igenom 24 729 sammanfattningar från vetenskapliga artiklar. Hittills har 536 artiklar bedömts som potentiellt relevanta för frågan och granskats i fulltext av två projektledare. Av dessa bedömdes 19 artiklar vara relevanta för SBU:s fråga, det vill säga att de redovisar studier om behandling eller rehabilitering vid postcovid, med de avgränsningar som SBU har satt upp (Avsnitt 2.1 Inklusionskriterier). Efter sedvanlig granskning av risken för systematiska fel i resultaten (risk för bias) har 8 av dessa artiklar bedömts ha hög risk för bias och 11 artiklar har bedömts ha måttlig risk för bias (Figur 3.1). De 11 artiklar som bedömts vara tillräckligt välgjorda (måttlig risk för bias) beskrivs kortfattat i Kapitel 4.
4. Resultat
4.1 Sammanfattning av resultaten
SBU har identifierat 19 studier om behandling eller rehabilitering vid postcovid. Av dessa studier har sju undersökt behandling eller rehabilitering av patienter med nedsatt lungfunktion eller andning [9] [10] [11] [12] [13] [14] [15], tre har undersökt patienter med lukt- eller smakbortfall [16] [17] [18], två studier har undersökt patienter med hjärntrötthet/kognitiv nedsättning [19] [20], en studie har undersökt patienter med depression eller ångest (posttraumatisk stress) [21] och ytterligare en har undersökt patienter med smärta [22]. Förutom detta identifierades även fem studier [23] [24] [25] [26] [27] som inte passade in i någon av de 13 symtomgrupper (Tabell 1.1) som representerar vanliga rapporterade symtom vid postcovid i Sverige. Av dessa 19 studier bedömdes åtta studier ha allt för hög risk för bias och beskrivs därför inte här. Dessa åtta studier finns ändå tillgängliga för den intresserade läsaren via evidenskartan, www.sbu.se/328#karta.
Nedan beskrivs kortfattat de elva studier som SBU bedömt vara relevanta och tillräckligt välgjorda (måttlig risk för bias). För mer detaljerade beskrivningar hänvisas till listan över relevanta studier. Det är studier från Kina [12] [16] [21] [28], Belgien [18], Italien [17] [20] [22], Storbritannien [9] [11] och Turkiet [10]. I fem studier har patienterna vårdats på sjukhus [12 [20] [21] [22] [23], i tre studier har patienterna fått någon form av öppenvård [10] [16] [18] och i tre studier bestod patientgruppen av både sjukhusvårdade och öppenvårdade patienter [9] [11] [17]. I de inkluderade studierna deltog totalt 1 175 patienter, könsfördelningen varierade (från 19 % till 70 % män) och medelåldern varierade mellan 42 och 59 år. Ingen av de inkluderade studierna handlade om barn.
Endast två av de inkluderade studierna var så lika i sin utformning att resultaten kunde slås samman. Sammantaget har tillförlitligheten till alla resultat från studierna bedömts vara mycket låg, vilket innebär att det inte går att bedöma effekten av någon av de beskrivna behandlingarna eller rehabiliterande åtgärderna.
4.1.1 Evidenskartan
Ett flertal olika symtom har rapporterats som en följd av covid-19 [6]. För att kunna redovisa resultatet överskådligt har 13 kategorier av symtomgrupper listats i tabellform i den så kallade levande evidenskartan som publicerats under uppdragstiden (Figur 4.1 och SBU:s webbplats, www.sbu.se/328#karta). Dessa kategorier har hämtats från de grupper av vanliga symtom eller besvär som i Patientregistret har angetts tillsammans med ICD-10-koden U09.9, Postinfektiöst tillstånd efter covid-19 (postcovid) [29]. Det är breda kategorier över både symtom och diagnoser, som ger en bild av vilka typer av långvariga följder som varit så allvarliga att vård har behövts.
I följande avsnitt beskrivs kortfattat de relevanta och tillräckligt välgjorda (måttlig risk för bias) studierna per kategori av symtom. Närmare beskrivningar ges på engelska under relevanta studier och artiklarna finns tillgängliga via evidenskartan på SBU:s webbsida, www.sbu.se/328#karta.
4.2 Lungfunktion och andning
4.2.1 Beskrivning av ingående studier
SBU har identifierat fyra randomiserade kontrollerade studier som har undersökt olika behandlingar för patienter som fortfarande besväras av nedsatt lungfunktion efter genomgången infektion, samtliga med måttlig risk för bias [9] [10] [11] [12].
Den ena studien (120 deltagare) undersökte effekten av ett 6-veckors långt hembaserat telerehabiliteringsprogram (TERECO) för patienter som tidigare vårdats på sjukhus för covid-19. Deltagarna uppnådde inte kriterierna enligt WHO:s definition på postcovid, men de påbörjade behandling efter genomgången infektion, hade symtom från andningsvägarna och uppföljningen skedde minst tre månader efter insjuknandet. Programmet bestod av olika typer av andningsövningar, samt konditions- och muskelträning. Behandlingen jämfördes med deltagare som enbart fått en kortare rådgivande insats vid undersökningens början. Det primära utfallsmåttet var den sträcka patienten kunde gå på 6 minuter (6 min walking distance, 6MWD) [12].
Den andra studien (281 deltagare) undersökte effekten av ett 8-veckors långt hembaserat träningsprogram med inspiratorisk muskelträning för patienter som främst besvärades av andfåddhet. Deltagarna hade rekryterats via sociala medier, stödgrupper för covid-19 och vid utskrivning från sjukhus. Deltagarna uppnådde kriterierna enligt WHO:s definition på postcovid. Andningsövningarna gjordes med hjälp av ett redskap för motståndsandning (Inspiratory Muscle Training, IMT) tre gånger per vecka. Det primära utfallsmåttet var hälsorelaterad livskvalitet. Behandlingen jämfördes med deltagare som fått sedvanlig vård [9].
Den tredje studien (52 deltagare) undersökte effekten av ett 5-veckors långt hembaserat träningsprogram med olika andningsövningar för patienter med andningsbesvär. Deltagarna uppnådde inte kriterierna enligt WHO:s definition på postcovid, men de påbörjade behandlingen efter genomgången infektion, hade symtom och uppföljningen skedde minst tre månader efter insjuknandet. Övningarna genomfördes med stöd av en sjuksköterska via telefonkontakt. De primära utfallsmåtten var lungfunktion (spirometri), och den sträcka patienten kunde gå på 6 minuter (6 min walking test, 6MWT). Behandlingen jämfördes med deltagare som enbart fått en broschyr med instruktioner [10].
Den fjärde studien (150 deltagare) undersökte effekten av 6-veckors handledd andningsträning online med hjälp av sångteknik. Deltagarna uppnådde kriterierna enligt WHO:s definition på postcovid. Det primära utfallsmåttet var hälsorelaterad livskvalitet. Behandlingen jämfördes med deltagare som fick sedvanlig vård [11].
4.2.2 Sammanvägda resultat och bedömning av tillförlitlighet
De fyra inkluderade studierna bedömdes vara för olika för att resultaten skulle kunna vägas samman. Dels är det oklart om de undersökta populationerna är tillräckligt lika då vissa vårdats på sjukhus och andra rekryterats oavsett svårighetsgrad på den tidigare covid-19-infektionen. Vad gäller behandlingar så har två studier undersökt liknande behandlingar [9] [10], men har använt olika utfallsmått. Den jämförande behandling som kontrollgrupperna har fått skiljer sig mellan studierna. Det vetenskapliga underlaget för varje behandling består av en enskild studie och därför har inga studieresultat kunnat sammanvägas. Samtliga resultat bedömdes ha mycket låg tillförlitlighet. Det går därför inte att bedöma effekten av någon av behandlingarna.
| 1 Måttlig risk för bias; 2 Få deltagare, få händelser; 3 Resultaten har inte upprepats; 4 Måttligt antal deltagare. MWD = Minute walking distance; MWT = Minute walk test; RCT = Randomised controlled trial; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs kontroll | Antal deltagare (Antal studier och studiedesign) [Referens] |
Utfall | Resultatets tillförlitlighet Avdrag |
| Telerehabilitering vs korta pedagogiska instruktioner | 120 (1 RCT) [12] |
Gångsträcka 6 minuter (6MWD) | Mycket låg Risk för bias −11 Precision −12 Överförbarhet −13 |
| Inspiratorisk muskelträning vs sedvanlig vård | 281 (1 RCT) [9] |
Hälsorelaterad livskvalitet (tre domäner: psykologisk, andfåddhet och aktivitet, symtom från bröstkorg) | Mycket låg Risk för bias −11 Precision −14 Överförbarhet −13 |
| Handledda andningsövningar via telemedicin vs broschyr som beskriver samma andningsövningar | 52 (1 RCT) [10] |
Spirometri, gångsträcka 6 minuter (6MWT) | Mycket låg Risk för bias −11 Precision −22 Överförbarhet −13 |
| Handledd andningsträning med hjälp av sångteknik (online) vs sedvanlig vård | 150 (1 RCT) [11] |
Hälsorelaterad livskvalitet | Mycket låg Risk för bias −11 Precision −1 2 Överförbarhet −13 |
4.3 Posttraumatisk stress
SBU har identifierat en studie som har undersökt behandling för patienter som beväras av posttraumatisk stress efter covid-19. Posttraumatisk stressyndrom har inte lyfts fram som vanligt förekommande i Patientregistret [29]. Men det är en psykiatrisk diagnos enligt ICD-10 (F43.1) och redovisas därför i evidenskartan under symtom- och diagnosgruppen depression och ångest (ICD-10 F32-34, F40-42). Studien är en randomiserad kontrollerad studie (n = 111) som bedömdes ha måttlig risk för bias. Studien undersökte om en kognitiv beteendeterapi med fokus på minnen av trauma (Narrative Exposure Therapi, NET) hade effekt på patienter som uppvisat symtom för posttraumatisk stress i samband med utskrivning från sluten vård. Deltagarna uppnådde inte kriterierna enligt WHO:s definition på postcovid, men de påbörjade behandling efter genomgången infektion, hade symtom och uppföljningen skedde minst tre månader efter insjuknandet. Behandlingen varade i åtta veckor (en till två sessioner per vecka) och jämfördes mot patienter som behandlats med annan individanpassad psykologisk behandling en gång i veckan. Det primära utfallsmåttet var symtom på posttraumatisk stress [21].
4.3.1 Sammanvägda resultat och bedömning av tillförlitlighet
Det vetenskapliga underlaget för interventionen består av en enskild studie. Resultatet bedömdes ha mycket låg tillförlitlighet. Det går därför inte att bedöma effekten av interventionen.
| 1 Måttlig risk för bias; 2 Få deltagare, få händelser; 3 Resultaten har inte upprepats. NET = Narrativ exponeringsterapi; RCT = Randomised controlled trial; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs kontroll | Antal deltagare (Antal studier och studiedesign) [Referens] |
Utfall | Resultatets tillförlitlighet Avdrag |
| Narrativ exponeringsterapi (NET) & individanpassad psykologisk behandling vs individanpassad psykologisk behandling | 111 (1 RCT) [21] |
Posttraumatisk stress | Mycket låg Risk för bias −11 Precision −12 Överförbarhet −13 |
4.4 Lukt- och smakbortfall
SBU har identifierat tre studier som har undersökt behandling för patienter med lukt- eller smakbortfall efter genomgången infektion, samtliga med måttlig risk för bias [16] [17] [18].
Den första studien är en icke-randomiserad kontrollerad studie (27 deltagare) som undersökte oral kortisonbehandling (metylprednisolon) under tio dagar och luktträning under tio veckor, jämfört med enbart luktträning [18]. Deltagarna uppnådde inte kriterierna enligt WHO:s definition på postcovid, men de påbörjade behandling efter genomgången infektion, hade symtom och uppföljningen skedde minst tre månader efter insjuknandet. Det primära utfallsmåttet var luktfunktion.
Den andra studien var en randomiserad kontrollerad studie (12 deltagare) som undersökt effekten av palmitoyletanolamid och luteolin (oralt) kombinerat med luktträning under 30 dagar, jämfört med enbart luktträning [16]. Deltagarna uppnådde kriterierna enligt WHO:s definition på postcovid. Det primära utfallsmåttet var luktfunktion.
Den tredje studien undersökte samma behandling som ovan (185 deltagare) under 90 dagar, jämfört med luktträning och placebo [17]. Deltagarna uppnådde kriterierna enligt WHO:s definition på postcovid. Det primära utfallsmåttet var luktfunktion.
4.4.1 Sammanvägda resultat och bedömning av tillförlitlighet
Två studier bedömdes vara tillräckligt lika för att vägas samman. Den ena studien är den pilotstudie [16] som genomfördes inför den större studien [17]. Interventionen i den tredje studien [18] bedömdes vara för olik för att vägas samman med de andra två. Det vetenskapliga underlaget för en intervention består alltså av två studier varav en är en pilotstudie och för den andra interventionen av en enskild studie. Samtliga resultat bedömdes ha mycket låg tillförlitlighet. Det går därför inte att bedöma effekten av någon av interventionerna.
| 1 Måttlig risk för bias; 2 Få deltagare, få händelser;3 Resultaten har inte upprepats; 4 Resultaten har endast upprepats som pilotstudie av samma forskargrupp. NRSI = Non-randomised studies of interventions; RCT = Randomised controlled trial; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs kontroll | Antal deltagare (Antal studier och studiedesign) [Referens] |
Utfall | Resultatets tillförlitlighet Avdrag |
| Palmitoyletanolamid och Luteolin (oralt) kombinerat med luktträning vs luktträning | 185 + 12 deltagare (2 RCT) [16] [17] |
Luktfunktion | Mycket låg Risk för bias −11 Precision −12 Överförbarhet −14 |
| Kortikosteroider (metylprednisolon) kombinerat med luktträning vs luktträning | 27 deltagare (1 NRSI prospective) [18] |
Luktfunktion | Mycket låg Risk för bias −11 Precision −22 Överförbarhet −13 |
4.5 Smärta
SBU har identifierat en studie med måttlig risk för bias som hade undersökt behandling för patienter med bland annat smärta i muskler och leder efter genomgången infektion.
Studien var en randomiserad kontrollerad studie (60 deltagare) som undersökte behandling med acetyl-L-karnitin (initialt intramuskulärt i 10 dagar, därefter oralt i 40 dagar) i kombination med rehabiliteringsträning, jämfört med enbart rehabiliteringsträning [22]. Deltagarna uppnådde inte kriterierna enligt WHO:s definition på postcovid, men de påbörjade behandling efter genomgången infektion, hade symtom och uppföljningen skedde minst tre månader efter insjuknandet. De primära utfallsmåtten var upplevd smärta och andfåddhet.
4.5.1 Sammanvägda resultat och bedömning av tillförlitlighet
Det vetenskapliga underlaget för interventionen består av en enskild studie. Resultatet bedömdes ha mycket låg tillförlitlighet. Det går därför inte att bedöma effekten av interventionen.
| 1 Måttlig risk för bias; 2 Få deltagare, få händelser; 3 Resultaten har inte upprepats. RCT = Randomised controlled trial; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs kontroll | Antal deltagare (Antal studier och studiedesign) [Referens] |
Utfall | Resultatets tillförlitlighet Avdrag |
| Acetyl-L-karnitin kombinerat med rehabiliteringsträning vs rehabiliteringsträning | 60 (1 RCT) [22] |
Upplevd smärta och andfåddhet | Mycket låg Risk för bias −11 Precision −22 Överförbarhet −13 |
4.6 Hjärntrötthet och kognitiv nedsättning
SBU har identifierat en studie med måttlig risk för bias som hade undersökt behandling för patienter med kognitiv nedsättning efter genomgången infektion.
Studien var en icke-randomiserad studie med matchade kontroller (45 deltagare) som undersökte kognitiv träning under två månader, jämfört med ingen träning [20]. Deltagarna uppnådde inte kriterierna enligt WHO:s definition på postcovid, men de påbörjade behandling efter genomgången infektion, hade symtom och uppföljningen skedde minst tre månader efter insjuknandet. Det primära utfallsmåttet var kognitiv funktion.
4.6.1 Sammanvägda resultat och bedömning av tillförlitlighet
Det vetenskapliga underlaget för interventionen består av en enskild studie. Resultatet bedömdes ha mycket låg tillförlitlighet. Det går därför inte att bedöma effekten av interventionen.
| 1 Måttlig risk för bias; 2 Få deltagare, få händelser; 3 Resultaten har inte upprepats. NRSI = Non-randomised studies of interventions; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs kontroll | Antal deltagare (Antal studier och studiedesign) [Referens] |
Utfall | Resultatets tillförlitlighet Avdrag |
| Kognitiv träning vs ingen behandling | 45 (1 NRSI) [20] |
Kognitiv funktion | Mycket låg Risk för bias −11 Precision −22 Överförbarhet −13 |
4.7 Övrigt
SBU har identifierat en studie som har undersökt behandling för patienter som inte passade in i någon av de 13 symtomgrupperna (Tabell 1.1).
Studien var en randomiserad kontrollerad studie (131 deltagare) med måttlig risk för bias som hade undersökt ett kinesiskt örtbaserat läkemedel (Bufei Huoxue) [23]. Behandlingen gavs i nittio dagar och jämfördes mot placebo. Studien utfördes på patienter som vårdats på sjukhus för covid-19. Av materialet är det svårt att utläsa vilka symtom patienterna faktiskt uppvisat vid studiestart, men enligt författarna har samtliga deltagare bevärats av ”qi-deficiency” (beskrivet av andra som bland annat allmän trötthet, andfåddhet med mera [30]). För övrigt verkar deltagarna uppnå kriterierna enligt WHO:s definition på postcovid (dvs. uppvisa kvarvarande eller nya symtom efter genomgången infektion minst 3 månader från insjuknande, där symtomen varat i minst 2 månader och inte kan förklaras av annan diagnos). De primära utfallsmåtten var den sträcka patienten kunde gå på 6 minuter (6 min walking distance, 6MWD) och lungförändringar.
4.7.1 Sammanvägda resultat och bedömning av tillförlitlighet
Det vetenskapliga underlaget för interventionen består av en enskild studie. Resultatet bedömdes ha mycket låg tillförlitlighet. Det går därför inte att bedöma effekten av interventionen.
| 1 Måttlig risk för bias; 2 Få deltagare, få händelser; 3 Resultaten har inte upprepats, och populationen har selekterats på andra kriterier än i svensk kontext, främst diagnosen ’qi deficiency’. MWD = Minute walking distance; RCT = Randomised controlled trial; SOF = Summary of findings; vs = Versus |
|||
| Intervention vs kontroll | Antal deltagare (Antal studier och studiedesign) [Referens] |
Utfall | Resultatets tillförlitlighet Avdrag |
| Kinesiskt örtbaserat läkemedel (Bufei Huoxue) vs placebo | 131 (1 RCT) [23] |
Lungförändringar (datortomografi), gångsträcka 6 min (6MWD) | Mycket låg Risk för bias −11 Precision −12 Överförbarhet −23 |
5. Diskussion
Enligt SBU:s bedömning har bara ett fåtal tillräckligt välgjorda (måttlig risk för bias) studier om behandling eller rehabilitering av patienter med postcovid publicerats. Dessa har undersökt behandling eller rehabilitering vid försämrad lungfunktion eller andningsbesvär [9] [10] [11] [12], posttraumatisk stress [21], lukt- eller smakförsämring [16] [17] [18], smärta [22], hjärntrötthet eller kognitiv nedsättning [20] och allmän trötthet med mera (övrigt) [23].
I dessa studier har patienterna antingen vårdats på sjukhus under den akuta fasen av covid-19 eller fått vård inom öppen vården. Det förkom även blandade grupper av patienter, som antingen fått vård på sjukhus eller av öppen vård. Flertalet studier har bara undersökt ett fåtal patienter och med relativt kort uppföljningstid. Eventuella könsskillnader har inte uppmärksammats, men ett par studier nämner att ingen skillnad i behandlingsrespons kunde påvisas mellan män och kvinnor [16] [17].
SBU har inte identifierat någon studie som handlar om behandling för barn med långvariga symtom efter covid-19. Trots detta har uppgifter från Socialstyrelsen visat att det är fler barn än vuxna som drabbats av multisystemiskt inflammatoriskt syndrom [4]. Det är dock ett tillstånd som inte är strikt relaterat till postcovid, eftersom det även kan förekomma under pågående infektion.
Sammantaget har det vetenskapliga underlaget mycket låg tillförlitlighet. Det går alltså inte att bedöma om någon av de studerade behandlingarna är effektiv eller inte, utifrån det underlag som identifierats fram till och med den 1 juni 2022. Det betyder inte att behandlingarna inte har någon effekt, men att det behövs fler välgjorda studier för att kunna bedöma effekten.
5.1 Begränsningar
Det finns ännu bara ett litet antal studier som undersökt behandling och rehabilitering vid postcovid. I denna sammanställning har vi inkluderat studier där deltagarna haft postcovid enligt WHO:s definition vid behandlingsstart, eller då det vid uppföljningen av behandlingen gått tillräckligt lång tid för att deltagarna skulle kunna matcha WHO:s definition av postcovid. Det kan därmed finnas studier som undersökt symtom liknande postcovid, men där behandling och uppföljning skett närmre inpå infektionen. Enligt WHO:s definition på postcovid handlar de studierna snarare om prevention av postcovid och dessa ingår inte i denna utvärdering.
Flera av de inkluderade studierna var små och i en del fall angavs att resultaten skulle betraktas som underlag för kommande studier.
Studier utan kontrollgrupp har inte inkluderats i denna utvärdering, eftersom det i sådana studier inte går att veta om förändringen beror på till exempel ett spontant tillfrisknande.
5.2 Beprövad erfarenhet
Trots att det finns få publicerade studier om behandling, så finns både svenska och internationella rekommendationer om hur patienter bör omhändertas vid långvariga symtom efter covid-19. Rekommendationerna är vanligen baserade på erfarenheter från behandling vid andra tillstånd med liknande symtom, den växande förståelsen för sjukdomen covid-19 och konsensusdiskussioner. Till exempel betonas vikten av att göra en helhetsbedömning för att utesluta eventuella andra orsaker till symtomen, att vården ska ges efter behov, att primärvården har en central roll, att multidisciplinär vård kan vara nödvändig samt att uppföljning och rehabilitering är viktigt, inte minst för patienter som har drabbats av svår eller kritisk covid-19 [31] [32] [33] [34].
Beprövad erfarenhet är ofta en viktig del i vårdens arbete, inte minst vid avsaknad av vetenskapligt underlag. Men, det är också ett oklart begrepp, när det gäller vilken erfarenhet som egentligen åberopas.
5.3 Att publicera resultat kontinuerligt
Covid-19 är en ny sjukdom och föremål för en hög forskningsaktivitet för frågor om till exempel förekomst av symtom, behandling, rehabilitering och preventiva åtgärder. Socialstyrelsen har publicerat preliminära uppgifter hämtade från Patientregistret över vilka symtom som är vanligast förekommande vid postcovid i Sverige (se uppgifter från juni 2022 i Tabell 1.1). Denna lista är preliminär eftersom uppgifter från primärvården inte rapporteras till Patientregistret, vilket även påtalats tidigare [6] [34] [35]. Det finns också en så kallad levande översikt (en rapport som ska uppdateras) från Region Örebro om symtom efter covid-19 [36].
För att ny kunskap om behandling och rehabilitering vid långvariga effekter efter covid-19 skulle vara tillgänglig för vården och andra intresserade aktörer har SBU sedan augusti år 2021 kontinuerligt publicerat information inom ramen för detta uppdrag. SBU har under uppdragstiden fått synpunkter från olika personer inom svensk sjukvård om att den kontinuerliga uppdateringen av ny publicerad vetenskaplig litteratur om behandling och rehabilitering vid postcovid har upplevts som värdefull.
Att systematiskt söka litteratur i internationella databaser varje vecka och hantera informationen kräver särskilda resurser för arbetet samt en organisatorisk uthållighet. På grund av det ständigt växande innehållet ställer det också föränderliga krav på hur resultatet kan presenteras både översiktligt och visuellt. Vid avslutningen av detta uppdrag har endast ett fåtal vetenskapliga artiklar publicerats om behandling eller rehabilitering vid postcovid, men med tanke på den pågående forskningsaktiviteten är det rimligt att förvänta sig avsevärt att mer kunskap publiceras under de kommande åren.
5.4 Avslutat uppdrag
Eftersom uppdraget nu har slutförts kommer informationen inte längre att uppdateras och evidenskartan är inte längre ”levande”. Däremot finns länkar till de vetenskapliga artiklarna via den slutliga evidenskarta som finns på SBU:s webbplats https://www.sbu.se/328. På webbplatsen finns även länkar till internationella levande översikter om behandling, rehabilitering, förekomst av symtom vid covid-19 och pågående studier inom området.
5.5 Framtida forskning och fortsatt arbete
Inga studier om behandling eller rehabilitering för barn med långvariga symtom efter covid-19 har hittills identifierats av SBU. Det talar för att det behövs en fortsatt uppmärksamhet när det gäller pågående forskning och eventuella kommande vetenskapliga artiklar om barn som drabbats av postcovid.
Kunskapen växer om symtom vid postcovid, där en interaktion mellan symtom och behandling kan påverka patientens situation. Ett exempel är patienter med betydande sömnsvårigheter efter covid-19, som också får en påverkan på den kognitiva förmågan. Om en patient har kognitiva besvär till följd av sömnsvårigheter (eller på grund av smärta eller läkemedelsbehandling) är det inte troligt att en rehabilitering som främst är inriktad på kognitiv funktion kommer att ha en effekt på symtomen. Framtida studier om behandling och rehabilitering vid postcovid behöver ta hänsyn till potentiella interaktioner mellan olika symtom och behandlingar. I rapporteringen av vetenskapliga studier är det också väsentligt att beskriva till exempel vilka symtom patienterna haft, hur lång tid symtomen varat och tidpunkten för insjuknande i covid-19.
De inkluderade studierna riktar sig till framför allt till patienter med specifika symtom, som till exempel andfåddhet. Det är troligt att samma symtom hos olika patienter kan ha olika underliggande orsaker, vilket i sin tur rimligen bör leda till olika behandlingar. En utmaning är att, i takt med en växande kunskap om underliggande mekanismer för långvariga symtom efter covid-19, bedriva studier där behandlingen riktas mot orsaken till symtomen.
Det finns utfall som inte har studerats i de studier som inkluderats i den här rapporten, till exempel tiden till återgång i arbete för vuxna och arbetsföra personer. För kommande forskning om behandling och rehabilitering kan utfallsmått med fördel hämtas från den internationella klassifikationen av funktionstillstånd, funktionshinder och hälsa (ICF) [37].
För att främja kunskapsutvecklingen har SBU under år 2021 undersökt vilka forskningsfrågor som prioriteras av personer som drabbats av postcovid, deras anhöriga, vårdpersonal samt personer som forskar inom området [38]. Det har gjorts med hjälp av metoden James Lind Alliance (JLA), som innebär att patienter och vårdpersonal tillsammans kommer överens om de viktigaste forskningsfrågorna inom ett område. Svar på inledande enkäter erhölls från drygt 300 patienter, cirka 20 anhöriga, drygt 40 vårdpersonal och cirka 20 forskare. I den slutliga prioriteringen deltog totalt 29 personer, varav 9 patienter. Frågorna rangordnas med den viktigaste frågan överst. Av tio prioriterade frågor var dessa tre de högst prioriterade:
- Vilken behandling hjälper mot långvariga neurologiska symtom samt kognitiva besvär vid covid-19?
- Vilken behandling är effektivast mot långvarig nedsättning i andningsförmåga?
- Vad är orsaken till att vissa personer utvecklar långvariga symtom vid covid-19?
Myndigheten för vård- och omsorgsanalys har ett pågående regeringsuppdrag om att kartlägga eventuella regionala skillnader i vård och omsorg av patienter med postcovid, som ska slutredovisas i oktober år 2022 [8].
6. Medverkande
6.1 Projektgrupp
6.1.1 Sakkunniga
- Judith Bruchfeld, specialist i infektionssjukdomar, överläkare, docent, Karolinska universitetssjukhuset, NPO Infektionssjukdomar
- Alison Godbolt, specialist i rehabiliteringsmedicin, överläkare, doctor of medicin, Danderyds sjukhus, NPO Rehabilitering, habilitering, försäkringsmedicin
- Kristina Hedman, legitimerad psykolog, specialist i neuropsykologi, Sundsvalls sjukhus, NPO Psykisk hälsa
- Olof Hertting, specialist i barn och ungdomsmedicin, överläkare, medicine doktor, Astrid Lindgrens barnsjukhus, NPO Barn och ungdomars hälsa
- Jörgen Månsson, specialist i allmänmedicin, adj. professor, Göteborgs universitet, NPO Nationellt primärvårdsråd
- Michael Runold, specialist i lung- och allergisjukdomar, överläkare, medicine doktor, Karolinska universitetssjukhuset, NPO Lung- och allergisjukdomar
- Marcus Ståhlberg, specialist i kardiologi, docent, Karolinska universitetssjukhuset, NPO Hjärt- och kärlsjukdomar
6.1.2 Bindningar och jäv
Sakkunniga har i enlighet med SBU:s krav lämnat deklarationer om bindningar och jäv. SBU har bedömt att de förhållanden som redovisats där är förenliga med myndighetens krav på saklighet och opartiskhet.
6.1.3 Kansli
- Elizabeth Åhsberg, projektledare
- Nathalie Peira, biträdande projektledare
- André Sjöberg, biträdande projektledare (t.o.m. december år 2021)
- Jessica Dagerhamn, biträdande projektledare (från november år 2021)
- Idha Kurtsdotter, biträdande projektledare (fr.o.m. april 2022)
- Maria Ahlberg, projektadministratör
- Carl Gornitzki, informationsspecialist (t.o.m. november år 2021)
- Hanna Olofsson, informationsspecialist (fr.o.m. november år 2021)
- Susanne Eksell, webbprojektledare
- Projektansvarig chef: Pernilla Östlund, Irene Edebert (tillförordnad chef fr.o.m. november år 2021).
6.2 Myndighetsrepresentanter
6.2.1 Socialstyrelsen
Anders Berg, projektledare
Malin Åman, utredare
6.2.2 Vetenskapsrådet
Abraham Mellkvist-Roos, forskningssekreterare
6.3 SBU:s vetenskapliga råd
- Svante Twetman, Köpenhamns universitet, ordförande (tandvård)
- Christel Bahtsevani, Malmö universitet, vice ordförande (omvårdnad)
- Magnus Svartengren, Uppsala universitet (arbetsmiljö)
- Ulrik Kihlbom, Uppsala universitet (etik)
- Lars Sandman, Linköpings universitet (etik)
- Magnus Tideman, Högskolan Halmstad (funktionshinderområdet)
- Pernilla Åsenlöf, Uppsala universitet (fysioterapi)
- Martin Henriksson, Linköpings universitet (hälsoekonomi)
- Katarina Steen Carlsson, Lunds universitet (hälsoekonomi)
- Jan Holst, Malmö och Lunds universitet (medicin)
- Mussie Msghina, Örebro universitet (medicin)
- Britt-Marie Stålnacke, Umeå universitet (medicin)
- Sverker Svensjö, Falun och Uppsala universitet (medicin)
- Anna Ehrenberg, Falun, Högskolan Dalarna (omvårdnad)
- Ata Ghaderi, Uppsala, Karolinska institutet (psykologi)
- Martin Bergström, Lunds universitet (socialt arbete)
- Lena Dahlberg, Falun, Högskolan Dalarna (socialt arbete)
- Christina Nehlin-Gordh, Uppsala universitet (socialt arbete)
- Sten-Åke Stenberg, Stockholms universitet (socialt arbete)
7. Referenser
- Socialstyrelsen. Postcovid-stöd till personal i och beslutsfattare i hälso- och sjukvården (del 1). Stockholm: Socialstyrelsen; 2021 2021-3-7276. [accessed June 20 2022]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2021-3-7276.pdf.
- Socialstyrelsen. Kodning vid covid-19. Koder ur ICD-10-SE och KVÅ samt information om DRG (2021-06-17). Stockholm: Socialstyrelsen; 2021 Version 3.7. [accessed June 20 2022]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/dokument-webb/klassifikationer-och-koder/kodning-av-covid-19.pdf.
- Vetenskapsakademin. Postakut Covid-19-syndrom – långtidskomplikationer av Covid-19. Kungliga vetenskapsakademins expertgrupp för covid-19; 2021. [accessed June 20 2022]. Available from: https://www.kva.se/sv/vetenskap-i-samhallet/halsa/halsa-expertgrupp-covid-19.
- Socialstyrelsen. Statistik om covid-19. 2021. [accessed June 20 2022]. Available from: https://www.socialstyrelsen.se/statistik-och-data/statistik/statistik-om-covid-19/.
- WHO. A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021. [accessed June 20 2022]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
- SBU. Långvariga symtom vid covid-19. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2020. SBU Bereder 319. [accessed June 20 2022]. Available from: https://www.sbu.se/319.
- SBU. Utvärdering av metoder i hälso- och sjukvården och insatser i socialtjänsten: en metodbok. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2020. Available from: https://www.sbu.se/metodbok.
- Myndigheten för Vård- och omsorgsanalys. Postcovid under utredning. Delrapport om vården och omsorgen av personer med postcovid. Myndigheten för vård- och omsorgsanalys, (MVO); 2022. [accessed June 20 2022]. Available from: https://www.vardanalys.se/rapporter/postcovid-under-utredning/.
- McNarry MA, Berg RMG, Shelley J, Hudson J, Saynor ZL, Duckers J, et al. Inspiratory Muscle Training Enhances Recovery Post COVID-19: A Randomised Controlled Trial. The European respiratory journal. 2022. Available from: https://doi.org/10.1183/13993003.03101-2021.
- Okan F, Okan S, Duran Yucesoy F. Evaluating the Efficiency of Breathing Exercises via Telemedicine in Post-Covid-19 Patients: Randomized Controlled Study. Clin Nurs Res. 2022;31(5):771-81. Available from: https://doi.org/10.1177/10547738221097241.
- Philip KEJ, Owles H, McVey S, Pagnuco T, Bruce K, Brunjes H, et al. An online breathing and wellbeing programme (ENO Breathe) for people with persistent symptoms following COVID-19: a parallel-group, single-blind, randomised controlled trial. The Lancet Respiratory medicine. 2022. Available from: https://doi.org/10.1016/S2213-2600(22)00125-4.
- Li Ja, Xia W, Zhan C, Liu S, Yin Z, Wang J, et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): a randomised controlled trial. Thorax. 2022;77(7):697-706. Available from: https://doi.org/10.1136/thoraxjnl-2021-217382.
- Martin I, Braem F, Baudet L, Poncin W, Fizaine S, Aboubakar F, et al. Follow-up of functional exercise capacity in patients with COVID-19: It is improved by telerehabilitation. Respir Med. 2021;183:106438. Available from: https://doi.org/10.1016/j.rmed.2021.106438.
- Mayer KP, Parry SM, Kalema AG, Joshi RR, Soper MK, Steele AK, et al. Safety and Feasibility of an Interdisciplinary Treatment Approach to Optimize Recovery From Critical Coronavirus Disease 2019. Critical care explorations. 2021;3(8):e0516. Available from: https://doi.org/10.1097/CCE.0000000000000516.
- Dun Y, Liu C, Ripley-Gonzalez JW, Liu P, Zhou N, Gong X, et al. Six-month outcomes and effect of pulmonary rehabilitation among patients hospitalized with COVID-19: a retrospective cohort study. Ann Med. 2021;53(1):2099-109. Available from: https://doi.org/10.1080/07853890.2021.2001043.
- D'Ascanio L, Vitelli F, Cingolani C, Maranzano M, Brenner MJ, Di Stadio A. Randomized clinical trial "olfactory dysfunction after COVID-19: olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin": preliminary results. Eur Rev Med Pharmacol Sci. 2021;25(11):4156-62. Available from: https://doi.org/10.26355/eurrev_202106_26059.
- Di Stadio A, D'Ascanio L, Vaira LA, Cantone E, De Luca P, Cingolani C, et al. Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo-Controlled Clinical Trial. Curr Neuropharmacol. 2022. Available from: https://doi.org/10.2174/1570159X20666220420113513.
- Le Bon SD, Konopnicki D, Pisarski N, Prunier L, Lechien JR, Horoi M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur Arch Otorhinolaryngol. 2021. Available from: https://doi.org/10.1007/s00405-020-06520-8.
- Hawkins J, Hires C, Keenan L, Dunne E. Aromatherapy Blend of Thyme, Orange, Clove Bud, and Frankincense Boosts Energy Levels in Post-COVID-19 Female Patients: A Randomized, Double-Blinded, Placebo Controlled Clinical Trial. Complement Ther Med. 2022. Available from: https://doi.org/10.1016/j.ctim.2022.102823.
- Palladini M, Bravi B, Colombo F, Caselani E, Di Pasquasio C, D'Orsi G, et al. Cognitive remediation therapy for post-acute persistent cognitive deficits in COVID-19 survivors: A proof-of-concept study. Neuropsychol Rehabil. 2022:1-18. Available from: https://doi.org/10.1080/09602011.2022.2075016.
- Fan Y, Shi Y, Zhang J, Sun D, Wang X, Fu G, et al. The Effects of Narrative Exposure Therapy on COVID-19 Patients with Post-Traumatic Stress Symptoms: A Randomized Controlled Trial. J Affect Disord. 2021. Available from: https://doi.org/10.1016/j.jad.2021.06.019.
- Scaturro D, Vitagliani F, Di Bella VE, Falco V, Tomasello S, Lauricella L, et al. The Role of Acetyl-Carnitine and Rehabilitation in the Management of Patients with Post-COVID Syndrome: Case-Control Study. Applied Sciences. 2022;12(8):4084-. Available from: https://doi.org/10.3390/app12084084.
- Chen Y, Liu C, Wang T, Qi J, Jia X, Zeng X, et al. Efficacy and safety of Bufei Huoxue capsules in the management of convalescent patients with COVID-19 infection: A multicentre, double-blind, and randomised controlled trial. J Ethnopharmacol. 2021:114830. Available from: https://doi.org/10.1016/j.jep.2021.114830.
- Scherlinger M, Pijnenburg L, Chatelus E, Arnaud L, Gottenberg JE, Sibilia J, et al. Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study. Vaccines. 2022;10(1). Available from: https://doi.org/10.3390/vaccines10010046.
- Wisnivesky JP, Govindarajulu U, Bagiella E, Goswami R, Kale M, Campbell KN, et al. Association of Vaccination with the Persistence of Post-COVID Symptoms. J Gen Intern Med. 2022. Available from: https://doi.org/10.1007/s11606-022-07465-w.
- Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022;70(1):61-7. Available from: https://doi.org/10.1136/jim-2021-002051.
- An X, Peng B, Huang X, Jiang H, Xiong Ze, Zhang H, et al. Ludangshen oral liquid for treatment of convalescent COVID-19 patients: a randomized, double-blind, placebo-controlled multicenter trial. Chin Med. 2022;17(1):42. Available from: https://doi.org/10.1186/s13020-022-00602-x.
- Chen H, Shi H, Liu X, Sun T, Wu J, Liu Z. Effect of Pulmonary Rehabilitation for Patients With Post-COVID-19: A Systematic Review and Meta-Analysis. Frontiers in medicine. 2022;9:837420. Available from: https://doi.org/10.3389/fmed.2022.837420.
- Socialstyrelsen. Statistik om tillstånd efter covid-19. 2021 2021-4-7353. [accessed June 20 2022]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2021-4-7353.pdf.
- Zhang Y, Zhang L, Zhao X, Liu Y, Du S. Symptom characteristics and prevalence of qi deficiency syndrome in people of varied health status and ages: A multicenter cross-sectional study. Journal of Traditional Chinese Medical Sciences.2(3):173-82. Available from: https://doi.org/10.1016/j.jtcms.2016.01.017.
- Vårdprogram. Nationellt vårdprogram för misstänkt och bekräftad covid-19, version 3.4. Svenska Infektionsläkarföreningen, Svenska Hygienläkarföreningen och Föreningen för Klinisk Mikrobiologi; 2021. [accessed June 20 2022]. Available from: https://infektion.net/wp-content/uploads/2021/03/nationella-covid-feb-2021-revision-210301.pdf.
- NICE. COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: National Insitute for Health and Care Excellence (NICE); 2022. [updated 25 januari 2022; accessed June 20 2022]. Available from: https://www.nice.org.uk/guidance/ng188.
- Nurek M, Clare Rayner, Anette Freyer, Sharon Taylor, Linn Järte, Nathalie MacDermott, et al. Recommendations for the recognition, diagnosis, and management of long COVID: a Delphi study. Br J Gen Pract. 2021. Available from: https://doi.org/https://doi.org/10.3399/BJGP.2021.0265.
- Socialstyrelsen. Om övergångar mellan sluten vård och öppen vård och omsorg. Stockholm: Socialstyrelsen; 2017 2017-1-13. [accessed June 20 2022]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2017-1-13.pdf.
- Socialstyrelsen. Uppföljning av primärvård och omställningen till en mer nära vård. Deluppdrag 1 - Nationell insamling av registeruppgifter från primärvården. Stockholm: Socialstyrelsen; 2021 2021-2-7233. [accessed June 20 2022]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2021-2-7223.pdf.
- Li M, Ahlzen R, Breimer L, Snellman A, Olsson L. Symtom efter den akuta fasen av covid-19 vs rapporterade av covid-19 negativa kontroller – en levande systematisk översikt. HTA-rapport 2022:49. HTA-enheten i CAMTÖ; 2022. [accessed June 20 2022]. Available from: https://www.regionorebrolan.se/contentassets/7480ad2d4b024d4f892eea63c8cb130f/2022.49-symtom-efter-den-akuta-fasen-av-covid-19-vs-rapporterade-av-covid-19-negativa-kontroller.pdf
- Socialstyrelsen. Internationell klassifikation av funktionstillstånd och hälsa (ICF). 2022 2022-1-7716. Available from: https://www.socialstyrelsen.se/statistik-och-data/klassifikationer-och-koder/icf/.
- SBU. Inventering och prioritering av forskningsfrågor gällande långvariga symtom vid covid19 (postcovid). Prioritering baserad på James Lind Alliance metod. Stockholm: Statens beredning för medicinsk och social utvärdering (SBU); 2021. Prioritering av vetenskapliga kunskapsluckor 324. [accessed June 20 2022]. Available from: www.sbu.se/324.
Relevant studies
Depression/Anxiety
Fan 2021
| Fan Y, Shi Y, Zhang J, Sun D, Wang X, Fu G, et al. The Effects of Narrative Exposure Therapy on COVID-19 Patients with Post-Traumatic Stress Symptoms: A Randomized Controlled Trial. J Affect Disord. 2021;293:141–7. Available from https://doi.org/10.1016/j.jad.2021.06.019 | |
| Country | China |
| Population | 111 patients (mean age±SD: 46 ± 12.34 years) with COVID-19 who isolated for 40.72 days on average, most of them with mild symptoms, near the discharge stage with positive screening results for posttraumatic stress symptoms (PTSS). |
| Treatment | The intervention group recived narrative Exposure Therapy (NET) and personalized psychological treatment. The NET therapy hade a duration of eight weeks with one or two sessions a week, lasting for 90-120 minutes each. The control only recived personalized psychological treatment. |
| Aim | Screen for the prevalence of PTSS among pre-discharged COVID-19 patients and explore the effects of NET on patients experiencing PTSS. |
| Outcomes | Post-traumatic stress symptoms (PTSD Checklist-Civilian version), Depression (The Self-rating Depression Scale, SDS), anxiety (The Self-rating Anxiety Scale, SAS) and Sleep quality (The Pittsburg Sleep Quality Index (PQSI). |
| Author's conclusion | "NET likely had a positive impact on PTSS of COVID-19 patients. Clinical staff should consider applying NET to improve the psychological well-being of patients who have experienced an epidemic such as COVID-19." |
| Risk of bias | Moderate |
Fever
No relevant studies.
Palpitations/POTS
No relevant studies.
Mental fatigue/Cognitive impairment
Palladini 2022
| Palladini M, Bravi B, Colombo F, Caselani E, Di Pasquasio C, D'Orsi G, et al. Cognitive remediation therapy for post-acute persistent cognitive deficits in COVID-19 survivors: A proof-of-concept study. Neuropsychol Rehabil. 2022:1-18. Available from https://doi.org/10.1080/09602011.2022.2075016 | |
| Country | Italy |
| Population | 45 adults (age mean ±SD: intervention = 59.60 ± 10.00; control= 56.80 ± 7.09); COVID-19 survivors presenting cognitive impairments at one-month follow-up. |
| Treatment | Cognitive remediation therapy (CRT). |
| Aim | Our case-control study investigates the efficacy of a CRT programme administered to COVID-19 survivors in the post-acute phase of the illness. |
| Outcomes | Cognitive functions were evaluated with a neuropsychological screening test (Cognition in Schizophrenia (BACS)). |
| Author's conclusion | "Our results could pave the way to a plausible innovative treatment targeting cognitive impairments and ameliorating the quality of life of COVID-19 survivors." |
| Risk of bias | Moderate |
Chronic obstructive pulmonary disease / Asthma
No relevant studies.
Smell/Taste
D'Ascanio 2021
| D'Ascanio L, Vitelli F, Cingolani C, Maranzano M, Brenner MJ, Di Stadio A. Randomized clinical trial "olfactory dysfunction after COVID-19: olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin": preliminary results. Eur Rev Med Pharmacol Sci. 2021;25(11):4156 – 62. Available from https://doi.org/10.26355/eurrev_202106_26059 | |
| Country | Italy |
| Population | 12 outpatients (mean age = 42 years), with confirmed history of COVID-19 and suffering from anosmia/hyposmia ≥ 90 days after a negative COVID-19 nasopharyngeal swab. |
| Treatment | The intervention was daily treatment with PEA/Luteolin oral supplement in addition to ofactory training/stimulation with Sniffin’ Sticks administered twice every day (10-minute session) for 30 days. The control group did only recived olfactory training. |
| Aim | Our study investigated the efficacy of a supplement with Palmitoylethanolamide (PEA) and Luteolin to support recovery of olfaction in COVID-19 patients. |
| Outcomes | Evaluation of smell function by Sniffin Sticks, follow-up after 30 days of treatment. |
| Author's conclusion | "Treatment combining olfactory rehabilitation with oral supplementation with PEA and Luteolin was associated with improved recovery of olfactory function, most marked in those patients with longstanding olfactory dysfunction. Further studies are necessary to replicate these findings and to determine whether early intervention including olfactory rehabilitation and PEA+Luteolin oral supplement might prevent SARS-CoV-2 associated olfactory impairment." |
| Risk of bias | Moderate |
Di Stadio 2022
| Di Stadio A, D'Ascanio L, Vaira LA, Cantone E, De Luca P, Cingolani C, et al. Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo-Controlled Clinical Trial. Curr Neuropharmacol. 2022. Available from https://doi.org/10.2174/1570159X20666220420113513 | |
| Country | Italy |
| Population | 185 adults (43.5 + 14.6 years; 65% female); prior COVID-19; persistent olfactory impairment >6 months after negative SARS-CoV-2 testing; without prior history of olfactory dysfunction sinonasal disorders. |
| Treatment | Ultramicronized PEA-LUT 770 mg oral supplements plus olfactory training for 90 days. |
| Aim | To investigate recovery of olfactory function in patients treated with PEA-LUT oral supplements plus olfactory training versus olfactory training plus placebo. |
| Outcomes | Sniffin’Sticks assessments were used to test the patients at baseline and 90 days. |
| Author's conclusion | "Among individuals with olfactory dysfunction post-COVID-19, combining PEA-LUT with olfactory training resulted in greater recovery of smell than olfactory training alone." |
| Risk of bias | Moderate |
Le Bon 2021
| Le Bon SD, Konopnicki D, Pisarski N, Prunier L, Lechien JR, Horoi M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur Arch Otorhinolaryngol. 2021. Available from https://doi.org/10.1007/s00405-020-06520-8 | |
| Country | Belgium |
| Population | 27 participants (age mean ±SD: intervention = 42 ± 14, control = 44 ± 14), non-hospitalized patients with loss of smell due to COVID-19 and still dysosmic 5 weeks after having lost their sense of smell. |
| Treatment | Oral corticosteroids (10-day course of 32 mg of methylprednisolone once daily) and olfactory training (sniffing four odors from ’Smell Training. Kit’ for approximately 10 s each twice daily, for 10 weeks). |
| Aim | In this pilot study, we investigated the efficacy and the safety of oral corticosteroids and olfactory training as a treatment for patients with persistent olfactory dysfunction as a result of COVID-19. |
| Outcomes | "The 'Sniffin’ Sticks' battery test" |
| Author's conclusion | "This pilot study may suggest the combination of a short course of oral corticosteroids and olfactory training is safe and may be beneficial in helping patients with enduring dysosmia recover from olfactory loss due to COVID-19. There is a crucial need for further investigation with larger cohorts to corroborate these findings." |
| Risk of bias | Moderate |
Lung function/Breathing
Li 2021
| Li Ja, Xia W, Zhan C, Liu S, Yin Z, Wang J, et al. A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): a randomised controlled trial. Thorax. 2021. Available from https://doi.org/10.1136/thoraxjnl-2021-217382 | |
| Country | China |
| Population | 120 participants (age range = 18–74, mean±SD = 50.61±10.98) discharged after inpatient treatment for COVID-19, with remaining dyspnoea complaints. |
| Treatment | Telerehabilitation programme for COVID-19 (TERECO), unsupervised home-based for 6-weeks, comprising breathing control and thoracic expansion, aerobic exercise and LMS exercise, delivered via smartphone, and remotely monitored with heart rate telemetry. |
| Aim | To investigate superiority of a telerehabilitation programme for COVID-19 (TERECO) over no rehabilitation with regard to exercise capacity, lower limb muscle strength (LMS), pulmonary function, health-related quality of life (HRQOL) and dyspnoea. |
| Outcomes | Primary outcome: 6 min walking distance (6MWD). Secondary outcomes: squat time, pulmonary function, HRQOL, mMRC-dyspnoea. Assessed at 6 weeks (post-treatment) and 24 weeks (follow-up). |
| Author's conclusion | "This trial demonstrated superiority of TERECO over no rehabilitation for 6MWD, LMS, and physical HRQOL." |
| Risk of bias | Moderate |
McNarry 2021
| McNarry MA, Berg RMG, Shelley J, Hudson J, Saynor ZL, Duckers J, et al. Inspiratory Muscle Training Enhances Recovery Post COVID-19: A Randomised Controlled Trial. The European respiratory journal. 2022. Available from https://doi.org/10.1183/13993003.03101-2021 | |
| Country | UK |
| Population | 281 adults (46.6 ± 12.2 years; 88% female) recovering from self-reported COVID-19 (9.0 ± 4.2 months post-acute infection), primary symptom of breathlessness. |
| Treatment | Inspiratory Muscle Training, 3 unsupervised sessions/week for 8 weeks, with a handheld inspiratory flow resistive device that wirelessly syncs to a mobile device via an App to provide graphical biofeedback. |
| Aim | The aim of the current study was to investigate the potential rehabilitative role of inspiratory muscle training (IMT). |
| Outcomes | Health-related quality of life and breathlessness questionnaires, respiratory muscle strength and fitness; pre and post intervention. |
| Author's conclusion | "IMT may represent an important home-based rehabilitation strategy for wider implementation as part of COVID-19 rehabilitative strategies. Given the diverse nature of long-COVID, further research is warranted on the individual responses to rehabilitation - the withdrawal rate herein highlights that no one strategy is likely to be appropriate for all." |
| Risk of bias | Moderate |
Okan 2022
| Okan F, Okan S, Duran Yucesoy F. Evaluating the Efficiency of Breathing Exercises via Telemedicine in Post-Covid-19 Patients: Randomized Controlled Study. Clin Nurs Res. 2022:10547738221097241. Available from https://doi.org/10.1177/10547738221097241 | |
| Country | Turkey |
| Population | 52 adults (age mean ±SD: intervention = 48.85 ± 10.85 , control = 52.19 ± 14.84); after Covid-19 pneumonia; presented to the Chest Diseases Outpatient Clinic with dyspnea. |
| Treatment | Breathing exercise (respiratory control, pursed lip breathing, and diaphragmatic breathing exercises) 3 times a day for 5 weeks (one session performed via telemedicine each week). |
| Aim | The aim of the study was to evaluate the effectiveness of breathing exercises given by telemedicine in post-Covid-19 dyspneic individuals. |
| Outcomes | Primary: Spirometry (FVC, FEV1, FEV1/FVC Ratio, and MVV) and six-minute walk test (6MWT); Secondary: St George’s Respiratory Questionnaire (SGRQ) score. |
| Author's conclusion | "With breathing exercise training applied through telemedicine, improvements were observed in the pulmonary functions, quality of life, and exercise capacities of dyspneic post-Covid-19 individuals." |
| Risk of bias | Moderate |
Philip 2022
| Philip KEJ, Owles H, McVey S, Pagnuco T, Bruce K, Brunjes H, et al. An online breathing and wellbeing programme (ENO Breathe) for people with persistent symptoms following COVID-19: a parallel-group, single-blind, randomised controlled trial. The Lancet Respiratory medicine. 2022. Available from https://doi.org/10.1016/S2213-2600(22)00125-4 | |
| Country | UK |
| Population | 150 adults (age mean ±SD: intervention = 49 ± 12 , control = 50 ± 12); recovering from COVID-19, with ongoing breathlessness. |
| Treatment | The English National Opera Breathe programme, breathing retraining using singing techniques (6 weeks, online). |
| Aim | We assessed whether an online breathing and wellbeing programme improves health related quality-of-life (HRQoL) in people with persisting breathlessness following COVID-19. |
| Outcomes | Health related quality-of-life (assessed using the RAND 36-item short form survey instrument mental health composite (MHC) and physical health composite (PHC) scores). |
| Author's conclusion | "Our findings suggest that an online breathing and wellbeing programme can improve the mental component of HRQoL and elements of breathlessness in people with persisting symptoms after COVID-19". |
| Risk of bias | Moderate |
Neurological difficulties
No relevant studies.
Kidney problems
No relevant studies.
Pneumonia
No relevant studies.
Pain
Scaturro 2022
| Scaturro D, Vitagliani F, Di Bella VE, Falco V, Tomasello S, Lauricella L, et al. The Role of Acetyl-Carnitine and Rehabilitation in the Management of Patients with Post-COVID Syndrome: Case-Control Study. Applied Sciences. 2022;12(8):4084-. Available from https://doi.org/10.3390/app12084084 | |
| Country | Italy |
| Population | 60 adults (age mean ±SD= 58.7 ±5.4); after Covid-19 pneumonia; with clinical characteristics similar to fibromyalgia. |
| Treatment | L-acetyl-carnitine (ALC 500 mg) therapy and rehabilitation protocol for 10 days. |
| Aim | We evaluated the effectiveness of physical exercise, in association with L-acetyl-carnitine (ALC) therapy, in patients with Post-COVID syndrome, on musculoskeletal pain, dyspnea, functional capacity, quality of life, and depression. |
| Outcomes | "Perceived musculoskeletal pain and degree of dyspnea". |
| Author's conclusion | "We believe that the combination of physical exercise with ALC intake is a promising and effective treatment in the management of post-COVID syndrome, especially for the management of musculoskeletal pain and depression, as well as for improving quality of life." |
| Risk of bias | Moderate |
Sleeping problems
No relevant studies.
Dizziness/Nausea
No relevant studies.
Other
| Chen Y, Liu C, Wang T, Qi J, Jia X, Zeng X, et al. Efficacy and safety of Bufei Huoxue capsules in the management of convalescent patients with COVID-19 infection: A multicentre, double-blind, and randomised controlled trial. J Ethnopharmacol. 2021:114830. Available from https://doi.org/10.1016/j.jep.2021.114830 | |
| Country | China |
| Population | 131 patients (age mean ±SD: intervention = 54.16 ± 12.11 , control = 52.51 ± 12.31) hospitalized; meeting discharge standards; in the rehabilitation period after COVID-19 infection; with qi deficiency in the lung and spleen. |
| Treatment | Chinese medicine Bufei Huoxue capsules. |
| Aim | The present study aimed to evaluate the efficacy and safety of Bufei Huoxue in restoring the functional status and exercise tolerance of patients recovering from COVID-19. |
| Outcomes | Primary: Chest CT, 6-min Walk Distance. Secondary: Fatigue, St George's Respiratory Questionnaire, Dyspnea, Chinese medicine symptom complex score. |
| Author's conclusion | "Bufei Huoxue may exert strong rehabilitative effects on physiological activity in patients recovering from COVID-19, which may in turn attenuate symptoms of fatigue and improve exercise tolerance." |
| Risk of bias | Moderate |
Studies with high risk of bias
Relevant articles, but excluded after quality assessment due to high risk of bias.
| Study |
| An X, Peng B, Huang X, Jiang H, Xiong Ze, Zhang H, et al. Ludangshen Oral Liquid for Treatment of Convalescent COVID-19 Patients: A Randomized, Double-Blind, Placebo-Controlled Multicenter Trial (preprint) 2022. Available from https://doi.org/10.1186/s13020-022-00602-x |
| Dun Y, Liu C, Ripley-Gonzalez JW, Liu P, Zhou N, Gong X, et al. Six-month outcomes and effect of pulmonary rehabilitation among patients hospitalized with COVID-19: a retrospective cohort study. Ann Med. 2021;53(1):2099-109. Available from https://doi.org/10.1080/07853890.2021.2001043 |
| Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022;70(1):61-7. Available from https://doi.org/10.1136/jim-2021-002051 |
| Hawkins J, Hires C, Keenan L, Dunne E. Aromatherapy Blend of Thyme, Orange, Clove Bud, and Frankincense Boosts Energy Levels in Post-COVID-19 Female Patients: A Randomized, Double-Blinded, Placebo Controlled Clinical Trial. Complement Ther Med. 2022:102823. Available from https://doi.org/10.1016/j.ctim.2022.102823 |
| Martin I, Braem F, Baudet L, Poncin W, Fizaine S, Aboubakar F, et al. Follow-up of functional exercise capacity in patients with COVID-19: It is improved by telerehabilitation. Respir Med. 2021;183:106438. Available from https://doi.org/10.1016/j.rmed.2021.106438 |
| Mayer KP, Parry SM, Kalema AG, Joshi RR, Soper MK, Steele AK, et al. Safety and Feasibility of an Interdisciplinary Treatment Approach to Optimize Recovery From Critical Coronavirus Disease 2019. Crit Care Explor. 2021;3(8):e0516. Available from https://doi.org/10.1097/CCE.0000000000000516 |
| Scherlinger M, Pijnenburg L, Chatelus E, Arnaud L, Gottenberg JE, Sibilia J, Felten R. Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study. Vaccines 2022;10. Available from https://doi.org/10.3390/vaccines10010046 |
| Wisnivesky JP, Govindarajulu U, Bagiella E, Goswami R, Kale M, Campbell KN, et al. Association of Vaccination with the Persistence of Post-COVID Symptoms. J Gen Intern Med. 2022. Available from https://doi.org/10.1007/s11606-022-07465-w |
Potentially relevant studies in languages other than English
| Study |
| Baranova IV, Gumeniuk AF, Semenenko AI, Iliuk IA, Osypenko IP. Ozone therapy as a component of a comprehensive rehabilitation program for patients after polysegmental pneumonia associated with SARS-CoV2 infection. Zaporozhye Medical Journal. 2021;23(6):752-8. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1543017 |
| Fatuev OE. Rehabilitation of patients after a new coronavirus infection COVID-19 in a sanatorium-resort institution. Physical & Rehabilitation Medicine, Medical Rehabilitation. 2022;4(1):63-7. Available from https://journals.eco-vector.com/2658-6843/article/view/104442/80457/zh_CN |
| Kasyanenko K, Maltsev OV, Kozlov KV, Zhdanov KV, Seryi IF. Effect of azoximer bromide on the severity of clinical manifestations in patients after SARS-CoV-2 infection. Infektsionnye Bolezni. 2021;19(4):15-22. Available from https://www.elibrary.ru/item.asp?id=48036506 |
| Kutashov VA. Actovegin use in patients with cognitive impairment after coronavirus infection (COVID-19). Nevrologiya, neiropsikhiatriya, psikhosomatika. 2021;13(2):65‐72. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1248433 |
| Luo Z-H, Wang K-X, Zhang Y-L, Chen Z-Q, Chen B, Chen J, et al. [Thumb-tack needles based on "<ovid:i>Biaoben</ovid:i> acupoint compatibility" for sequela of COVID-19 during recovery period]. Zhongguo zhen jiu = Chinese acupuncture & moxibustion. 2022;42(3):281-6. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1737395 |
| 史锁芳, 方祝元, 熊侃, 叶德梁, 汪为民, 陈永昶, et al. Clinical Observation of the Rehabilitation Formula for Banking up Earth to Generate Metal in Treating COVID-19 Patients with Deficiency of Lung and Spleen Syndrome in the Recovery Stage. 南京中医药大学学报. 2020. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/czh-803 |
| Shatylko T, Gamidov S, Popova AY. Evaluating the effect of BESTFertil antioxidant complex on semen parameters and severity of asthenic syndrome in men with a recent history of novel coronavirus infection (COVID-19). (In Russ.). Andrology and Genital Surgery. 2021;22(4):68-76. Available from: https://doi.org/10.17650/1726-9784-2021-22-4-68-76 |
| Shogenova LV, Tuet TT, Kryukova NO, Yusupkhodzhaeva KA, Pozdnyakova DD, Kim TG, et al. KalHydrogen inhalation in rehabilitation program of the medical staff recovered from COVID-19. Cardiovascular Therapy and Prevention (Russian Federation). 2021;20(6):24-32. Available from https://doi.org/10.15829/1728-8800-2021-2986 |
| Wan XY, XianZe Li JunChang, Gong XiaoLi Liang, YuQing Gao, SongKai Xu JiPing, Yue XiaoQiang. Clinical effect of Guanggu jisheng decoction in treatment of recovery stage coronavirus disease 2019. Academic Journal of Second Military Medical University. 2020;41(8):813-7. Available from https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-859166 |
| Zolotovskaia IA, Shatskaia PR, Davydkin IL, Shavlovskaya OA. [Post-COVID-19 asthenic syndrome]. Zh Nevrol Psikhiatr Im S S Korsakova. 2021;121(4):25-30. Available from https://doi.org/10.17116/jnevro202112104125 |
Excluded studies
Articles that seemed relevant based on their abstracts, but later were excluded based on their full texts, as they did not meet the inclusion critera.
| Study | Main reason for exclusion |
| Handheld breathing device could reduce breathlessness and improve physical fitness in long COVID patients. Operating Theatre Journal. 2022(378):2-. | Wrong study design |
| Innovating in response to Long Covid. Frontline (20454910). 2022;28(2):48-51. | Wrong study design |
| Post COVID‐19 organizing pneumonia treated with mycophenolate mofetil. Respirology (Carlton, Vic). 2021;26:473-4. Available from https://doi.org/10.1111/resp.14150_969 | Wrong study design |
| Pulmonary function after nintedanib treatment in post‐COVID‐19 pulmonary fibrosis. Respirology (Carlton, Vic). 2021;26:94-5. Available from https://doi.org/10.1111/resp.14150_55 | Wrong study design |
| SSRIs show rapid effects in post‐COVID depression. Brown University Psychopharmacology Update. 2022;33(3):8-. Available from https://doi.org/10.1002/pu.30844 | Wrong study design |
| Women with long COVID-19 may need targeted rehabilitation to help counter problems with physical activity tolerance. Operating Theatre Journal 2021:20-20. | Wrong study design |
| Abdelalim AA, Mohamady AA, Elsayed RA, Elawady MA, Ghallab AF. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: A randomized controlled trial. Am J Otolaryngol. 2021;42(2):102884. Available from https://doi.org/10.1016/j.amjoto.2020.102884 | Wrong population |
| Abdelmaksoud AA, Ghweil AA, Hassan MH, Rashad A, Khodeary A, Aref ZF, et al. Olfactory Disturbances as Presenting Manifestation Among Egyptian Patients with COVID-19: Possible Role of Zinc. Biol Trace Elem Res. 2021;199(11):4101-8. Available from https://doi.org/10.1007/s12011-020-02546-5 | Wrong population |
| Abodonya, A. M., Abdelbasset, W. K., Awad, E. A., Elalfy, I. E., Salem, H. A., Elsayed, S. H. (2021). Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: A pilot control clinical study. Medicine, 100(13), e25339. Available from https://doi.org/10.1097/MD.0000000000025339 | Wrong population |
| Abreus Mora JL, González Curbelo VB, Mena Pérez O, Abreus Vázquez JA, Del Sol Santiago FJ, Bernal Valladares EJ. PHYSICAL REHABILITATION AND COVID-19. Universidad y Sociedad. 2022;14:172-83. Available from https://rus.ucf.edu.cu/index.php/rus/article/view/2620 | Wrong study design |
| Abuhelaiqa E, Alkadi MM, Khan S, Nauman A, Othman M, Al-Malki HA. Sustained low-efficiency dialysis vs. Continuous renal replacement therapy in critically ill COVID-19 Patients. J Am Soc Nephrol. 2021;32:105. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1489273 | Wrong study design |
| Acat M, Yildiz Gulhan P, Oner S, Turan MK. The performance of artificial intelligence supported Thoracic CT to evaluate the radiologic improvement in patients with COVID-19 pneumonia: comparision pirfenidon vs. corticosteroid. Int J Clin Pract. 2021:e14961. | Wrong population |
| Acosta-Dighero R, Rodriguez-Nunez I, Solis-Grant MJ, Torres-Castro R, Garcia-Soto C. Post COVID-19 rehabilitation: A current challenge. Rehabilitacion post COVID-19: un desafio vigente. 2020;148(10):1531-2. Available from https://doi.org/10.4067/S0034-98872020001001531 | Wrong study design |
| Affeldt S, Alcorn P, Duke T, Raynes E. Role of physical therapy in reducing length of stay and occurrence of post intensive care syndrome among COVID 19 patients admitted to the intensive care unit. The FASEB Journal. 2021;35. Available from https://doi.org/10.1096/fasebj.2021.35.S1.03232 | Wrong population |
| Agostini F, Mangone M, Ruiu P, Paolucci T, Santilli V, Bernetti A. Rehabilitation setting during and after Covid-19: An overview on recommendations. J Rehabil Med. 2021;53(1):jrm00141. Available from https://doi.org/10.2340/16501977-2776 | Wrong study design |
| Ahmed I, Inam AB, Belli S, Ahmad J, Khalil W, Jafar MM. Effectiveness of aerobic exercise training program on cardio-respiratory fitness and quality of life in patients recovered from COVID-19. Eur J Physiother. 2021. Available from https://doi.org/10.1080/21679169.2021.1909649 | Wrong study design |
| Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428-42. 2021;14(6):1672-3. Available from https://doi.org/10.1177/01410768211032850 | Wrong study design |
| Al Chikhanie Y, Veale D, Vergès S, Hérengt F. Suivi à 6 mois de patients post-COVID19 réanimés, intubés et réhabilités. Revue des Maladies Respiratoires Actualités 2022;14:181-81. Available from https://doi.org/10.1016/j.rmra.2021.11.313 | Wrong study design |
| Alawna M, Amro M, Mohamed AA. Aerobic exercises recommendations and specifications for patients with COVID-19: a systematic review. Eur Rev Med Pharmacol Sci. 2020;24(24):13049-55. Available from https://doi.org/10.26355/eurrev_202012_24211 | Wrong population |
| Albu S, Rivas Zozaya N, Murillo N, Garcia-Molina A, Figueroa Chacon CA, Kumru H. Multidisciplinary outpatient rehabilitation of physical and neurological sequelae and persistent symptoms of covid-19: a prospective, observational cohort study. Disabil Rehabil. 2021:1-8. Available from https://www.tandfonline.com/doi/full/10.1080/09638288.2021.1977398 | Wrong control |
| Alcazar-Navarrete B, Molina Paris J, Martin Sanchez FJ. Management and Follow up of Respiratory Patients in the Post-COVID-19 Era: Are We Ready Yet? Seguimiento del paciente con enfermedad respiratoria en la era post-COVID-19: estamos preparados? 2020;56(10):685-6. Available from https://doi.org/10.1016/j.arbr.2020.08.005 | Wrong study design |
| Alenskaya TL. Innovative methods of rehabilitation at the outpatient and homestages in patients after pneumonia covid-19. Meditsinskiy Sovet. 2021;2021(4):220-9. Available from https://doi.org/10.21518/2079-701X-2021-4-220-229 | Wrong study design |
| Alexandre F, Castanyer A, Vernet A, Aliaga-Parera JL, Oliver N, Oliver N, et al. Late Breaking Abstract - Effects of pulmonary rehabilitation on major symptoms of long COVID (post-COVID-19 syndrome): preliminary results. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.PA3896 | Wrong study design |
| Alizadeh S, Taklavi S, Alilou MM, Feizipour H. The effectiveness of existential therapy on death anxiety and meaning of life in recovered patients of COVID-19. Urmia Medical Journal. 2021;32(5):388-98. Available from http://umj.umsu.ac.ir/article-1-5557-en.html | Wrong population |
| AlZaben M, Al Adwan F. The Effectiveness of a Counselling Program in Reducing the Death Anxiety and Improving Self-Efficacy Among a Sample of Female Middle-Aged Teachers Recovered from COVID-19 Virus. Omega. 2022:302228221086704. Available from https://doi.org/10.1177/00302228221086704 | Wrong population |
| Ambrosino P, Molino A, Calcaterra I, Formisano R, Stufano S, Spedicato GA, et al. Clinical Assessment of Endothelial Function in Convalescent COVID-19 Patients Undergoing Multidisciplinary Pulmonary Rehabilitation. Biomedicines. 2021;9(6). Available from https://doi.org/10.3390/biomedicines9060614 | Wrong study design |
| An X, Duan L, Zhang YH, Jin D, Zhao S, Zhou RR, et al. The three syndromes and six Chinese patent medicine study during the recovery phase of COVID-19. Chin Med. 2021;16(1):44. Available from https://doi.org/10.1186/s13020-021-00454-x | Wrong study design |
| An YW, Yuan B, Wang JC, Wang C, Liu TT, Song S, et al. Clinical characteristics and impacts of traditional Chinese medicine treatment on the convalescents of COVID-19. Int J Med Sci. 2021;18(3):646-51. Available from https://doi.org/10.7150/ijms.52664 | Wrong population |
| Andina-Martinez D, Alonso-Cadenas JA, Cobos-Carrascosa E, Bodegas I, Oltra-Benavent M, Plazaola A, et al. SARS-CoV-2 acute bronchiolitis in hospitalized children: neither frequent nor more severe. Pediatr Pulmonol. 2021. Available from https://doi.org/10.1002/ppul.25731 | Wrong intervention |
| Andrenelli E, Negrini F, de Sire A, Lazzarini SG, Patrini M, Ceravolo MG, et al. Rehabilitation and COVID-19: update of the rapid living systematic review by Cochrane Rehabilitation Field as of October 31st, 2021. Eur J Phys Rehabil Med. 2022. Available from https://doi.org/10.23736/S1973-9087.22.07434-2 | Wrong study design |
| Antoniou KM, Vasarmidi E, Russell A-M, Andrejak C, Crestani B, Delcroix M, et al. European Respiratory Society Statement on Long COVID-19 Follow-Up. The European respiratory journal 2022. Available from https://doi.org/10.1183/13993003.02174-2021 | Wrong study design |
| Arentz S, Hunter J, Khamba B, Mravunac M, Lee Z, Alexander K, et al. Honeybee products for the treatment and recovery from viral respiratory infections including SARS-COV-2: A rapid systematic review. Integrative medicine research. 2021:100779. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8483994/pdf/main.pdf | Wrong population |
| Arienti C, Kiekens C, Bettinsoli R, Engkasan JP, Frischknecht R, Gimigliano F, et al. Cochrane Rehabilitation: 2020 annual report. Eur J Phys Rehabil Med. 2021;57(2):303-8. Available from https://doi.org/10.23736/s1973-9087.21.06877-5 | Wrong study design |
| Asly M, Hazim A. Rehabilitation of post-COVID-19 patients. The Pan African medical journal. 2020;36:168. Available from https://doi.org/10.11604/pamj.2020.36.168.23823 | Wrong study design |
| Austelle C, Badran B, Huffman S, Dancy M, Kautz S, George M. At-home telemedicine controlled taVNS twice daily for 4 weeks reduces long COVID symptoms of anxiety and fatigue. Brain Stimul. 2021;14(6):1703. Available from https://doi.org/10.1016/j.brs.2021.10.368 | Wrong study design |
| Avancini A, Belluomini L, Benato G, Trestini I, Tregnago D, Menis J, et al. Exercise for counteracting post-acute COVID-19 syndrome in patients with cancer: an old but gold strategy? Acta Oncol. Department of Oncology, University of Verona Hospital Trust, Verona, Italy Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, Verona, Italy Philadelphia, Pennsylvania: Taylor & Francis Ltd; 2022. p. 388-92. Available from https://doi.org/10.1080/0284186X.2021.2009565 | Wrong study design |
| Ayoubkhani D, Bermingham C, Pouwels K, Glickman M, Nafilyan V, Zaccardi F, et al. Changes in the trajectory of Long Covid symptoms following COVID-19 vaccination: community-based cohort study (preprint). Available from https://doi.org/10.1101/2021.12.09.21267516 | Wrong study design |
| Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ (Clinical research ed). 2022;377:e069676. Available from https://doi.org/10.1136/bmj-2021-069676 | Wrong population |
| Azzolino D, Passarelli PC, D’Addona A, Cesari M. Nutritional strategies for the rehabilitation of COVID-19 patients. Eur J Clin Nutr. 2021;75(4):728-30. Available from https://doi.org/10.1038/s41430-020-00795-0 | Wrong study design |
| Babliuk L, Fediaeva S, Babova I, Mesoedova V, Tamazlykar S. Rehabilitation of post-COVID patients with chronic fatigue and cognitive disorders syndromes. Balneo and Prm Research Journal. 2022;13(1):9-. Available from https://doi.org/10.12680/balneo.2022.497 | Wrong study design |
| Bagri NK, Deepak RK, Meena S, Gupta SK, Prakash S, Setlur K, et al. Outcomes of multisystem inflammatory syndrome in children temporally related to COVID-19: a longitudinal study. Rheumatol Int. 2021. Available from https://doi.org/10.1007/s00296-021-05030-y | Wrong population |
| Baig M, Joo M, Nada KMSA, Deer R, Seashore J. Pulmonary Rehabilitation and Its Role in Long-Term COVID-19 Recovery. Am J Respir Crit Care Med. 2021;203(9). Available from https://doi.org/10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A4118 | Wrong study design |
| Baily-Scanlan C, Kehoe B, Moloney E. Implementation of a Virtual Pulmonary Rehabilitation Programme for patients with chronic respiratory disease in response to the COVID-19 pandemic. Ir. J. Med. Sci. 2021;190:192-92. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1576752 | Wrong study design |
| Bangash MN, Owen A, Alderman JE, Chotalia M, Patel JM, Parekh D. COVID-19 recovery: potential treatments for post-intensive care syndrome. The Lancet Respiratory medicine. 2020;8(11):1071-3. Available from https://doi.org/10.1016/S2213-2600(20)30457-4 | Wrong study design |
| Barbara C, Clavario P, De Marzo V, Lotti R, Guglielmi G, Porcile A, et al. Effects of exercise rehabilitation in patients with long COVID-19. European journal of preventive cardiology. 2022. Available from https://doi.org/10.1093/eurjpc/zwac019 | Wrong study design |
| Bari E, Ferrarotti I, Saracino L, Perteghella S, Torre ML, Richeldi L, et al. Mesenchymal stromal cell secretome for post-covid-19 pulmonary fibrosis: A new therapy to treat the long-term lung sequelae? Cells. 2021;10(5). Available from https://doi.org/10.3390/cells10051203 | Wrong study design |
| Baricich A, Borg MB, Cuneo D, Cadario E, Azzolina D, Balbo PE, et al. Midterm functional sequelae and implications in rehabilitation after COVID-19: a cross-sectional study. Eur J Phys Rehabil Med. 2021;57(2):199-207. Available from https://doi.org/10.23736/s1973-9087.21.06699-5 | Wrong study design |
| Barker-Davies, R. M., O'Sullivan, O., Senaratne, K., Baker, P., Cranley, M., Dharm-Datta, S., et al. (2020). The Stanford Hall consensus statement for post-COVID-19 rehabilitation. British journal of sports medicine, 2020;54(16), 949-59. Available from https://doi.org/10.1136/bjsports-2020-102596 | Wrong study design |
| Barrett C, Pelow L. A clinical audit to determine the outcome of inpatient exercise rehabilitation on outcomes including functional capacity, dyspnoea and muscle strength in patients diagnosed with COVID-19. Ir J Med Sci. 2021;190:S8-S. Available from https://irishthoracicsociety.com/eposter/a-clinical-audit-to-determine-the-outcome-of-inpatient-exercise-rehabilitation-on-outcomes-including-functional-capacity-dyspnoea-and-muscle-strength-in-patients-diagnosed-with-covid-19/ | Wrong study design |
| Barros A, Anderson Vajão Silva F, Araújo de Carvalho S. Atuação da fisioterapia respiratória em pacientes pós Covid-19: Uma revisão sistemática. Brazilian Journal of Health Review. 2021;4:24663-75. Available from: https://doi.org/10.34119/bjhrv4n6-084 | Wrong study design |
| Barros CMSS, Freire RS, Frota E, Rezende Santos AG, Farias MEL, Rodrigues MGA, et al. Short-Course of Methylprednisolone Improves Respiratory Functional Parameters After 120 Days in Hospitalized COVID-19 Patients (Metcovid Trial): A Randomized Clinical Trial. Frontiers in medicine 2021;8:758405. Available from https://doi.org/10.3389/fmed.2021.758405 | Wrong population |
| Basu D, Chavda VP, Mehta AA. Therapeutics for COVID-19 and post COVID-19 complications: An update. Current research in pharmacology and drug discovery 2022:100086. Available from https://doi.org/10.1016/j.crphar.2022.100086 | Wrong study design |
| Baum P, Bleckwenn M, Laufs U. [Diagnostics and treatment of post-covid-syndrome: a multidisciplinary approach]. Post-Covid-Syndrom: Wie diagnostizieren, wie behandeln? 2022;164:36-39. Available from https://doi.org/10.1007/s15006-021-0541-0 | Wrong study design |
| Bazdyrev E, Rusina P, Panova M, Novikov F, Grishagin I, Nebolsin V. Lung Fibrosis after COVID-19: Treatment Prospects. Pharmaceuticals (Basel) 2021;14. Available from https://doi.org/10.3390/ph14080807 | Wrong study design |
| Becker F, Laake JH, Hofso K. Rehabilitation after Covid-19. Tidsskr. Nor. Laegeforen. 2020;140:880-83. Available from https://doi.org/10.4045/tidsskr.20.0352 | Wrong study design |
| Belcaro G, Cornelli U, Cesarone MR, Scipione C, Scipione V, Hu S, et al. Preventive effects of Pycnogenol R on cardiovascular risk factors (including endothelial function) and microcirculation in subjects recovering from coronavirus disease 2019 (COVID-19). Minerva Med. 2021. Available from https://doi.org/10.23736/s0026-4806.21.07650-3 | Wrong population |
| Benzakour LBG. Update of the Potential Treatments for Psychiatric and Neuropsychiatric Symptoms in the Context of the Post-COVID-19 Condition: Still a Lot of Suffering and Many More Things to Learn. Trauma Care. 2022;2(2):131-50. Available from https://doi.org/10.3390/traumacare2020011 | Wrong study design |
| Bertolucci F, Sagliocco L, Tolaini M, Posteraro F. Comprehensive rehabilitation treatment for sub-acute COVID-19 patients: an observational study. Eur J Phys Rehabil Med. 2021;57(2):208-15. Available from https://doi.org/10.23736/s1973-9087.21.06674-0 | Wrong study design |
| Birch S, Alraek T, Grobe S. Reflections on the potential role of acupuncture and Chinese herbal medicine in the treatment of Covid-19 and subsequent health problems. Integrative medicine research. 2021;10:100780. Available from https://doi.org/10.1016/j.imr.2021.100780 | Wrong study design |
| Birtolo LI, Prosperi S, Monosilio S, Cimino S, Filomena D, Alfarano M, et al. Follow-up of hospitalized COVID-19 survivors: Assessment of short- and long-term cardiovascular sequelae after SARS-CoV-2 infection. European Heart Journal, Supplement 2021;23:G97. Available from https://doi.org/10.1093/eurheartj/suab135.039 | Wrong study design |
| Boglione L, Meli G, Poletti F, Rostagno R, Moglia R, Cantone M, et al. Risk factors and incidence of Long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect? QJM : monthly journal of the Association of Physicians. 2021. Available from https://doi.org/10.1093/qjmed/hcab297 | Wrong population |
| Bogolepova AN, Osinovskaya NA, Kovalenko EA, Makhnovich EV. Fatigue and cognitive impairment in post-COVID syndrome: possible treatment approaches. Nevrologiya, Neiropsikhiatriya, Psikhosomatika. 2021;13(4):88-93. Available from https://doi.org/10.14412/2074-2711-2021-4-88-93 | Wrong study design |
| Boisvert I, Bujold M, Saury S. État des connaissances - Pratiques visant à mesurer ou réduire les symptômes psychologiques des personnes qui présentent une affection post-COVID-19 2022. | Wrong study design |
| Bontsevich R, Vovk Y, Solovyova L. COVID-19: treatment of early chronic COVID syndrome. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.PA3674 | Wrong study design |
| Bordas-Martinez J, Luzardo-Gonzalez A, Arencibia A, Tormo F, Mateu L, Vicens-Zygmunt V, et al. Effects of Early Physical Therapy and Follow-Up in Acute Severe Coronavirus Disease 2019 Pneumonia: A Retrospective Observational Study. Frontiers in medicine. 2022;9:866055. Available from https://doi.org/10.3389/fmed.2022.866055 | Wrong population |
| Borg K, Stam H. Rehabilitation of post-Covid-19 syndrome – once again a call for action! Journal of Rehabilitation Medicine (Stiftelsen Rehabiliteringsinformation). 2021;53(1):1-. Available from https://doi.org/10.2340/16501977-2783 | Wrong study design |
| Botek M, Krejci J, Valenta M, McKune A, Sladeckova B, Konecny P, et al. Molecular Hydrogen Positively Affects Physical and Respiratory Function in Acute Post-COVID-19 Patients: A New Perspective in Rehabilitation. Int J Environ Res Public Health. 2022;19(4). Available from https://doi.org/10.3390/ijerph19041992 | Wrong population |
| Boutou AK, Asimakos A, Kortianou E, Vogiatzis I, Tzouvelekis A. Long COVID-19 Pulmonary Sequelae and Management Considerations. Journal of personalized medicine. 2021;11(9). Available from https://www.mdpi.com/2075-4426/11/9/838 | Wrong study design |
| Brennan A, Broughan JM, McCombe G, Brennan J, Collins C, Fawsitt R, et al. Enhancing the management of long COVID in general practice: a scoping review. BJGP open. 2022. Available from https://doi.org/10.3399/BJGPO.2021.0178 | Wrong population |
| Bressi B, Paltrinieri S, Fugazzaro S, Costi S. Letter to the editor: Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement Ther Clin Pract. 2021;43. Available from https://doi.org/10.1016/j.ctcp.2021.101368 | Wrong study design |
| Brodsky MB, Gilbert RJ. The Long-Term Effects of COVID-19 on Dysphagia Evaluation and Treatment. Arch Phys Med Rehabil. 2020;101(9):1662-4. Available from https://doi.org/10.1016/j.apmr.2020.05.006 | Wrong study design |
| Brugliera L, Spina A, Castellazzi P, Cimino P, Tettamanti A, Houdayer E, et al. Rehabilitation of COVID-19 patients. J Rehabil Med. 2020;52(4). Available from https://doi.org/10.2340/16501977-2678 | Wrong study design |
| Brugliera L, Spina A, Giordani A, Iannaccone S. Response to: Nutritional strategies for the rehabilitation of COVID-19 patients. Eur J Clin Nutr. 2021;75(4):731-2. Available from https://doi.org/10.1038/s41430-020-00801-5 | Wrong study design |
| Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, et al. Preliminary evidence on long COVID in children. Acta Paediatrica, International Journal of Paediatrics. 2021;110(7):2208-11. Available from https://doi.org/10.1111/apa.15870 | Wrong study design |
| Burnfield J, Votto J, Hays A, Stuart M, Lewis L, Prettyman E, et al. Six Minute Walk Test Changes during Long-Term Acute Care Hospital Rehabilitation for Patients Post COVID-19. Arch Phys Med Rehabil. 2022;103(3):e13-e4. Available from https://doi.org/10.1016/j.apmr.2022.01.036 | Wrong study design |
| Byambasukh O, Avirmed B, Shirmen B, Khasag A. Exercise intervention and the development of long COVID: A survey of patients admitted to the hospital in Mongolia. Journal of Diabetes Investigation. 2021;12:33. Available from https://doi.org/10.1111/jdi.13663 | Wrong study design |
| Büsching GZZSJ-PSTKR. Effectiveness of Pulmonary Rehabilitation in Severe and Critically Ill COVID-19 Patients: A Controlled Study. Int. J. Environ. Res. Public Health 2021;18:8956-56. Available from https://doi.org/10.3390/ijerph18178956 | Wrong control |
| Caballero-Garcia A, Perez-Valdecantos D, Guallar P, Caballero-Castillo A, Roche E, Noriega DC, et al. Effect of Vitamin D Supplementation on Muscle Status in Old Patients Recovering from COVID-19 Infection. Medicina (Kaunas). 2021;57(10). Available from https://doi.org/10.3390/medicina57101079 | Wrong population |
| Cadth. Post-COVID-19 condition: a condition-level review 2022. Available from https://www.cadth.ca/post-covid-19-condition-condition-level-review | Wrong study design |
| Cahalan R, Mockler S. SingStrong for Long Covid: A singing and breathing pilot intervention for respiratory symptoms and general health in Long Covid: A mixed-methods study. Ir. J. Med. Sci. 2021;190:200-00. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1576699 | Wrong study design |
| Cahalan RM, Meade C, Mockler S. SingStrong-A singing and breathing retraining intervention for respiratory and other common symptoms of long COVID: A pilot study. Canadian journal of respiratory therapy : CJRT = Revue canadienne de la therapie respiratoire : RCTR. 2022;58:20-7. Available from https://doi.org/10.29390/cjrt-2021-074 | Wrong study design |
| Camargo-Martínez, W., Lozada-Martínez, I., Escobar-Collazos, A., Navarro-Coronado, A., Moscote-Salazar, L., Pacheco-Hernández, A., Janjua, T., & Bosque-Varela, P. Post-COVID 19 neurological syndrome: Implications for sequelae's treatment. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia, 2021;88, 219-25. Available from https://doi.org/10.1016/j.jocn.2021.04.001 | Wrong intervention |
| Canter B, Weerahandi HM, Mak W, Raschen L, Burack O, Reinhardt J, et al. Rehabilitation intensity in covid-19 patients in a skilled nursing facility. J Am Geriatr Soc. 2021;69:S285. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-1194918 | Wrong study design |
| Carda S, Invernizzi M, Bavikatte G, Bensmail D, Bianchi F, Deltombe T, et al. The role of physical and rehabilitation medicine in the COVID-19 pandemic: The clinician's view. Ann Phys Rehabil Med. 2020;63(6):554-6. Available from https://doi.org/10.1016/j.rehab.2020.04.001 | Wrong study design |
| Carraro U, Albertin G, Martini A, Giuriati W, Guidolin D, Masiero S, et al. To contrast and reverse skeletal muscle weakness by Full-Body In-Bed Gym in chronic COVID-19 pandemic syndrome. Eur J Transl Myol. 2021;31(1). Available from https://doi.org/10.4081/ejtm.2021.9641 | Wrong study design |
| Carson E, Hemenway AN. A Scoping Review of Pharmacological Management of Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in 2021. Am J Ther. 2022;29(3):305-e321. Available from https://doi.org/10.1097/MJT.0000000000001486 | Wrong study design |
| Catalan IP, Marti CR, Sota DPdl, Alvarez AC, Gimeno MJE, Juana SF, et al. Corticosteroids for COVID-19 symptoms and quality of life at 1 year from admission. J Med Virol. 2022;94(1):205-10 Available from https://doi.org/10.1002/jmv.27296 | Wrong population |
| Ceban F, Leber A, Jawad MY, Yu M, Lui LMW, Subramaniapillai M, et al. Registered clinical trials investigating treatment of long COVID: a scoping review and recommendations for research. Infectious diseases (London, England). 2022:1-11. Available from https://doi.org/10.1080/23744235.2022.2043560 | Wrong study design |
| Centeno-Cortez AK, Diaz-Chavez B, Santoyo-Saavedra DR, Alvarez-Mendez PA, Pereda-Samano R, Acosta-Torres LS. [Respiratory physiotherapy in post-acute COVID-19 adult patients: Systematic review of literature]. Fisioterapia respiratoria en pacientes adultos post-COVID-19: revision sistematica de la literatura. 2022;60(1):59-66. Available from https://pubmed.ncbi.nlm.nih.gov/35271227/ | Wrong study design |
| Centers for Disease Control and Prevention. Patient History and Physical Exam: Evaluating and Caring for Patients with Post-COVID Conditions 2021. Available from https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-workup.html | Wrong study design |
| Centers for Disease Control and Prevention. Evaluating and Caring for Patients with Post-COVID Conditions: Interim Guidance 2021. Available from https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-index.html | Wrong study design |
| Centers for Disease Control and Prevention. General Clinical Considerations: Evaluating and Caring for Patients with Post-COVID Conditions 2021. Available from https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-clinical-eval.html | Wrong study design |
| Ceravolo MG, de Sire A, Andrenelli E, Negrini F, Negrini S. Systematic rapid "living" review on rehabilitation needs due to COVID-19: update to March 31st, 2020. Eur J Phys Rehabil Med. 2020;56(3):347-53. Available from https://doi.org/10.23736/S1973-9087.20.06329-7 | Wrong study design |
| Cesarone MR, Hu S, Belcaro G, Cornelli U, Feragalli B, Corsi M, et al. Pycnogenol R-Centellicum R supplementation improves lung fibrosis and post-COVID-19 lung healing. Minerva Med. 2022;113(1):135-40. Available from https://doi.org/10.23736/S0026-4806.20.07225-0 | Wrong population |
| Cha C, Baek G. Symptoms and management of long COVID: A scoping review. J Clin Nurs. 2021. Available from https://doi.org/10.1111/jocn.16150 | Wrong study design |
| Chaban O, Khaustova O, Assonov D. P.0370 Escitalopram efficacy in post-covid depression treatment: a pilot study. Eur. Neuropsychopharmacol. 2021;53:S270. Available from https://doi.org/10.1016/j.euroneuro.2021.10.350 | Wrong study design |
| Chandrashekar YYPC, Soumya SV, Sinitha SMSB, Madhu H. Efficacy of laser photodynamic therapy on fungal infections and post COVID mucormycosis: a narrative review. J. Cardiovasc. Dis. Res. 2021;12:407-16. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1374772 | Wrong study design |
| Charfeddine S, Ibn Hadjamor H, Torjmen S, Kraiem S, Hammami R, Bahloul A, et al. Sulodexide in the treatment of patients with long COVID 19 symptoms and endothelial dysfunction: The results of TUN-EndCOV study. Archives of Cardiovascular Diseases Supplements 2022;14:127-27. Available from https://doi.org/10.1016/j.acvdsp.2021.10.007 | Wrong study design |
| Charlotte N, Balaire X, Bardet A, Vial H, Asofii M, Biot V, et al. Practice of cardiac rehabilitation at the beginning of the COVID-19 pandemic: Challenges and responses. Archives of Cardiovascular Diseases Supplements 2022;14:117-17. Available from https://doi.org/10.1016/j.acvdsp.2021.09.265 | Wrong study design |
| Chaturvedi SK. Covid-19, Coronavirus and Mental Health Rehabilitation at Times of Crisis. Journal of Psychosocial Rehabilitation and Mental Health. 2020;7(1). Available from https://doi.org/10.1007/s40737-020-00162-z | Wrong study design |
| Chaudhry A, Master H. Top tips: managing long COVID. Guidelines in Practice. 2021;24(1):26-32. Available from https://www.guidelinesinpractice.co.uk/ infection/top-tips-managing-long-covid/455742.article | Wrong study design |
| Chen H, Shi H, Liu X, Sun T, Wu J, Liu Z. Effect of Pulmonary Rehabilitation for Patients With Post-COVID-19: A Systematic Review and Meta-Analysis. Frontiers in medicine. 2022;9:837420. Available from https://doi.org/10.3389/fmed.2022.837420 | Wrong study design |
| Chen JM, Wang ZY, Chen YJ, Ni J. The Application of Eight-Segment Pulmonary Rehabilitation Exercise in People With Coronavirus Disease 2019. Front Physiol. 2020;11. Available from https://doi.org/10.3389/fphys.2020.00646 | Wrong study design |
| Chishima Y, Huai-Ching Liu IT, A EW. Temporal distancing during the COVID-19 pandemic: letter writing with future self can mitigate negative affect. Applied psychology Health and well-being. 2021. Available from https://doi.org/10.1111/aphw.12256 | Wrong population |
| Chisman E, France S, McCormick S, Shardha J. THE LEEDS POST COVID-19 REHABILITATION PATHWAY;WHAT WE HAVE LEARNT AND ACHIEVED SO FAR. Br. J. Occup. Ther. 2021;84:1-1. Available from https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-1370062 | Wrong study design |
| Chitra SM, Mallika P, Anbu N, Narayanababu R, Sugunabai A, David Paul Raj RS, et al. An open clinical evaluation of selected siddha regimen in expediting the management of COVID-19 – a randomized controlled study. J Ayurveda Integr Med. 2021. Available from https://doi.org/10.1016/j.jaim.2021.01.002 | Wrong population |
| Christensen J, O'Callaghan K, Sinclair H, Hawke K, Love A, Hajkowicz K, et al. Risk factors, Treatment and Outcomes of Subacute Thyroiditis Secondary to COVID-19: A Systematic Review. Intern Med J. 2021;52(4):522-529. Available from https://doi.org/10.1111/imj.15432 | Wrong outcome |
| Chu MMH, Gopikrishna D, Rocke JPJ, Kumar BN. Implementing a covid-19 specialist smell clinic: Experience at the wrightington, wigan and leigh teaching hospitals (wwl), nhs foundation trust, united kingdom. Med J Malaysia. 2021;76:9-13. Available from http://www.e-mjm.org/2021/v76s4/COVID-19-specialist-smell-clinic.pdf | Wrong study design |
| Chung TW-H, Zhang H, Wong FK-C, Sridhar S, Chan K-H, Cheng VC-C, et al. Neurosensory Rehabilitation and Olfactory Network Recovery in Covid-19-related Olfactory Dysfunction. Brain sciences. 2021;11(6). Available from https://doi.org/10.3390/brainsci11060686 | Wrong study design |
| Clayton NA, Walker EF-SA. Clinical profile and recovery pattern of dysphagia in the COVID-19 patient: a prospective observational cohort within NSW. Aust. Crit. Care 2022. Available from https://doi.org/10.1016/j.aucc.2022.01.001 | Wrong study design |
| Coudeyre E, Cormier C, Costes F, Lefevre-Colau MM, Grolier M. Muscular rehabilitation post COVID-19 infection. Revue du Rhumatisme Monographies. 2021;88(3):251-254. Available from https://doi.org/10.1016/j.monrhu.2021.03.002 | Wrong population |
| Cox N, Holland A. Experiences of implementing a home-based pulmonary rehabilitation program during COVID-19. Respirology. 2022;27:38-. | Wrong study design |
| COVID-19 UPDATE. Virtual Post-Sepsis Recovery Program May Also Help Recovering COVID-19 Patients. RT: The Journal for Respiratory Care Practitioners, 2021;34(1), 9. | Wrong study design |
| Cui W, Ouyang T, Qiu Y, Cui D. Literature Review of the Implications of Exercise Rehabilitation Strategies for SARS Patients on the Recovery of COVID-19 Patients. Healthcare. 2021;9(5):590-. Available from https://doi.org/10.3390/healthcare9050590 | Wrong population |
| Curci, C., Pisano, F., Bonacci, E., Camozzi, D. M., Ceravolo, C., Bergonzi, R., De Franceschi, S., Moro, P., Guarnieri, R., Ferrillo, M., Negrini, F., & de Sire, A. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 Rehabilitation Unit and proposal of a treatment protocol. Eur J Phys Rehabil Med. 2020;56(5), 633-41. Available from https://doi.org/10.23736/S1973-9087.20.06339-X | Wrong study design |
| D'Amico F, Rossella DA. Risk of sarcopoenia and prevention of disability in post COVID 19 elderly patients. Bone Reports. 2021;14. Available from https://doi.org/10.1016/j.bonr.2021.100951 | Wrong study design |
| Dai S, Zhao B, Liu D, Zhou Y, Liu Y, Lan L, et al. Follow-Up Study of the Cardiopulmonary and Psychological Outcomes of COVID-19 Survivors Six Months After Discharge in Sichuan, China. Int J Gen Med. 2021;14:7207-17. Available from https://doi.org/10.2147/IJGM.S337604 | Wrong intervention |
| Damanti S, Ramirez GA, Bozzolo EP, Rovere-Querini P, De Lorenzo R, Magnaghi C, et al. Six-month respiratory outcomes and exercise capacity of COVID-19 acute respiratory failure patients treated with continuous positive airway pressure. Intern Med J. 2021;51(11):1810-5. Available from https://doi.org/10.1111/imj.15345 | Wrong study design |
| Danesh V, Arroliga AC, Bourgeois JA, Widmer AJ, McNeal MJ, McNeal TM. Post-acute sequelae of COVID-19 in adults referred to COVID recovery clinic services in an integrated health system in Texas. Proc (Bayl Univ Med Cent). 2021;34(6):645-8. Available from https://doi.org/10.1080/08998280.2021.1972688 | Wrong intervention |
| Dasgupta A, Kalhan A, Kalra S. Long term complications and rehabilitation of COVID-19 patients. JPMA The Journal of the Pakistan Medical Association. 2020;70(5):S131-S5. Available from https://pubmed.ncbi.nlm.nih.gov/32515393/ | Wrong study design |
| Davies P, Lillie J, Prayle A, Evans C, Griffiths B, du Pre P, et al. Association Between Treatments and Short-Term Biochemical Improvements and Clinical Outcomes in Post-Severe Acute Respiratory Syndrome Coronavirus-2 Inflammatory Syndrome. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2021;22:e285-e93. Available from https://doi.org/10.1097/PCC.0000000000002728 | Wrong population |
| Daynes E, Gerlis C, Chaplin E, Gardiner N, Singh SJ. Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition - A cohort study. Chron Respir Dis. 2021;18:14799731211015691. Available from https://doi.org/10.1177/14799731211015691 | Wrong study design |
| de Sire A, Andrenelli E, Negrini F, Lazzarini SG, Cordani C, Ceravolo MG, et al. Rehabilitation and COVID-19: update of the rapid living systematic review by Cochrane Rehabilitation Field as of February 28th, 2022. Eur J Phys Rehabil Med. 2022. Available from https://doi.org/10.23736/S1973-9087.22.07593-1 | Wrong study design |
| Dean E. Managing the effects of long-COVID. Nurs Stand. 2021;36(2):11-. | Wrong study design |
| Décary S, Dugas M, Stefan T, Langlois L, Skidmore B, Bhéreur A, et al. Care Models for Long COVID: A Rapid Systematic Review (preprint) 2021. Available from https://doi.org/10.1101/2021.11.17.21266404 | Wrong study design |
| del Valle MF, Valenzuela J, Marzuca-Nassr GN, Cabrera-Inostroza C, del Sol M, Lizana P, Escobar-Cabello, M, Muñoz-Cofre R. Eight Weeks of Supervised Pulmonary Rehabilitation Are Effective in Improving Resting Heart Rate and Heart Rate Recovery in Severe COVID-19 Patient Survivors of Mechanical Ventilation. Medicina. 2022;58(4):514-. Available from https://doi.org/10.3390/medicina58040514 | Wrong study design |
| Demeco, A., Marotta, N., Barletta, M., Pino, I., Marinaro, C., Petraroli, A., Moggio, L., & Ammendolia, A. Rehabilitation of patients post-COVID-19 infection: a literature review. The Journal of international medical research, 2020;48(8), 300060520948382. Available from https://doi.org/10.1177/0300060520948382 | Wrong population |
| De Souza Y, MacEdo J, Nascimento R, Alves MAM, Medeiros S, Leal L, et al. Low-Intensity Pulmonary Rehabilitation Through Videoconference for Post-Acute COVID-19 Patients. Am J Respir Crit Care Med. 2021;203(9). Available from https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A4124 | Wrong study design |
| Dhoonmoon L. Self-management for patients with suspected 'long COVID'. Independent Nurse. 2021;2021(2):22-4. Available from https://www.independentnurse.co.uk/clinical-article/self-management-for-patients-with-suspected-long-covid/234556/ | Wrong study design |
| Dhooria S, Chaudhary S, Sehgal IS, Agarwal R, Arora S, Garg M, et al. High-dose <ovid:i>versus</ovid:i> low-dose prednisolone in symptomatic patients with post-COVID-19 diffuse parenchymal lung abnormalities: an open-label, randomised trial (Acronym: COLDSTER). The European respiratory journal 2021. Available from https://doi.org/10.1183/13993003.02930-2021 | Wrong population |
| Ding H, He F, Lu YG, Hao SW, Fan XJ. Effects of non-drug interventions on depression, anxiety and sleep in COVID-19 patients: A systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(3):1087-96. Available from https://doi.org/10.26355/eurrev_202101_24679 | Wrong population |
| Diotallevi F, Mazzanti S, Properzi P, Olivieri S, Giacometti A, Offidani A. Is there a POST-COVID dermatological syndrome? The integrated dermato-infectious disease experience of a single centre. J Eur Acad Dermatol Venereol. 2022;36(3):e166-e9. Available from https://doi.org/10.1111/jdv.17803 | Wrong study design |
| Dixon MG, Lutfy C. Outcomes Among Patients Referred to Outpatient Rehabilitation Clinics After COVID-19 diagnosis - United States, January 2020-March 2021 (vol 70, pg 967, 2021). Mmwr-Morbidity and Mortality Weekly Report 2021;70:967-71. Available from https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-1372288 | Wrong control |
| Dmytriiev D, Dobrovanov O. Post-COVID pain syndrome. Anaesthesia, Pain and Intensive Care 2021;25:505-12. Available from https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/es/covidwho-1372227 | Wrong study design |
| Donaldson K, Brenton A, Haslam P, Turner N, Talbot J, Newsham J, et al. Delivering a community-based COVID-19 rehabilitation service using existing pulmonary rehabilitation teams is safe and feasible. Thorax. 2021;76:A103-A4. Available from https://doi.org/10.1136/thorax-2020-BTSabstracts.180 | Wrong study design |
| Dowds J, Sheill G, Brien KO, Murphy N, Bannan C, Martin-Loeches I. Profiling the physical rehabilitation of COVID-19 patients admitted to critical care. Intensive Care Medicine Experimental. 2020;8. Available from https://doi.org/10.1186/s40635-020-00354-8 | Wrong study design |
| Duan Q, Guo G, Ren Y, Shang H, Du J, Li M, et al. Treatment Outcomes, Influence Factors of 116 Hospitalized COVID-19 Patients with Longer/Prolonged Treatment Course in Wuhan, China; 2020. Available from https://doi.org/10.2139/ssrn.3550017 | Wrong population |
| Duncan DL. Living with long COVID. Journal of Prescribing Practice 2021;3:362-68. Available from https://doi.org/10.12968/jprp.2021.3.9.362 | Wrong study design |
| Duymaz T. Pulmonary Rehabilitation in Post-Acute Period of COVID-19 Infection: Prospective Randomized Controlled Trial; 2020. Available from https://clinicaltrials.gov/ct2/show/NCT04365738 | Wrong study design |
| Elanwar R, Hussein M, Magdy R, Eid RA, Yassien A, Abdelsattar AS, et al. Physical and Mental Fatigue in Subjects Recovered from COVID-19 Infection: A Case-Control Study. Neuropsychiatr Dis Treat. 2021;17:2063-71. Available from https://doi.org/10.2147/NDT.S317027 | Wrong study design |
| Ensinck G, Gregorio G, Flores RM, Crowe CI, Clerico Mosina P, Curi C, et al. [Consensus on treatment of multisystemic inflammatory syndrome associated with COVID-19]. Consenso sobre el tratamiento del sindrome inflamatorio multisistemico asociado a COVID-19. 2021;119:S198-S211. Available from https://doi.org/10.5546/aap.2021.S198 | Wrong study design |
| Everaerts S, Heyns A, Langer D, Beyens H, Hermans G, Troosters T, et al. COVID-19 recovery: benefits of multidisciplinary respiratory rehabilitation. BMJ open respiratory research 2021;8. Available from https://doi.org/10.1136/bmjresp-2020-000837 | Wrong study design |
| Falvey JR, Ferrante LE. Flattening the disability curve: Rehabilitation and recovery after COVID-19 infection. Heart & lung. 2020;49(5):440-1. Available from https://doi.org/10.1016/j.hrtlng.2020.05.001 | Wrong study design |
| Fan WH, Hin HJ. Effect of aerobics on the rehabilitation training of patients with COVID-19. Basic & Clinical Pharmacology & Toxicology. 2021;128:235-. | Wrong study design |
| Fekrazad R, Fekrazad S. The Potential Role of Photobiomodulation in Long COVID-19 Patients Rehabilitation. Photobiomodulation, photomedicine, and laser surgery. 2021;39(4):229-31. Available from https://doi.org/10.1089/photob.2020.4984 | Wrong study design |
| Fernández-de-Las-Peñas C, Martín-Guerrero JD, Cancela-Cilleruelo I, Moro-López-Menchero P, Rodríguez-Jiménez J, Navarro-Pardo E, et al. Exploring the Recovery Curves for Long-term Post-COVID Functional Limitations on Daily Living Activities: The LONG-COVID-EXP-CM Multicenter Study. The Journal of infection. 2022. Available from https://doi.org/10.1016/j.jinf.2022.01.031 | Wrong study design |
| Fernández-de-Las-Peñas C, Martín-Guerrero JD, Navarro-Pardo E, Cancela-Cilleruelo I, Moro-López-Menchero P, Pellicer-Valero OJ. Exploring Trajectory Curves from Loss of Smell and Taste in Previously Hospitalized COVID-19 Survivors: the LONG-COVID-EXP-CM Multicenter Study. J Gen Intern Med. 2022. Available from https://doi.org/10.1007/s11606-022-07459-8 | Wrong study design |
| Feshchenko YI, Ostrovskyy MM, Varunkiv OI, Horovenko NH. Improved quality of life and dyspnea with erdosteine in COVID-19 patients after hospital discharge. Minerva Respiratory Medicine. 2022;61(2):54-62. Available from https://doi.org/10.23736/S2784-8477.22.01992-1 | Wrong population |
| Fisher DL, Pavel A, Malnick S. Rapid recovery of taste and smell in a patient with SARS-CoV-2 following convalescent plasma therapy. QJM : monthly journal of the Association of Physicians. 2021;114(5):319-20. Available from https://doi.org/10.1093/qjmed/hcaa341 | Wrong study design |
| Foged F, Rasmussen IE, Bjorn Budde J, Rasmussen RS, Rasmussen V, Lyngbaek M, et al. Fidelity, tolerability and safety of acute high-intensity interval training after hospitalisation for COVID-19: a randomised cross-over trial. BMJ open sport & exercise medicine. 2021;7(3):e001156. Available from http://dx.doi.org/10.1136/bmjsem-2021-001156 | Wrong study design |
| Fontana LCC, Bernardo G, Bernardo CD, Vieira JD, Dias FM, Bom BM, et al. Results from a multidisciplinary rehabilitation program with patients post-COVID-19 infection. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.PA2118 | Wrong study design |
| Fowler-Davis S, Platts K, Thelwell M, Woodward A, Harrop D. A mixed-methods systematic review of post-viral fatigue interventions: Are there lessons for long Covid? PLoS One. 2021;16(11):e0259533. Available from https://doi.org/10.1371/journal.pone.0259533 | Wrong population |
| Frajkova Z, Tedla M, Tedlova E, Suchankova M, Geneid A. Postintubation Dysphagia During COVID-19 Outbreak-Contemporary Review. Dysphagia. 2020;35(4):549-57. Available from https://doi.org/10.1007/s00455-020-10139-6 | Wrong study design |
| Fugazzaro S, Contri A, Esseroukh O, Kaleci S, Croci S, Massari M, et al. Rehabilitation Interventions for Post-Acute COVID-19 Syndrome: A Systematic Review. Int J Environ Res Public Health. 2022;19(9). Available from https://doi.org/10.3390/ijerph19095185 | Wrong study design |
| Funke-Chambour M, Bridevaux P-O, Clarenbach CF, Soccal PM, Nicod LP, von Garnier C, et al. Swiss Recommendations for the Follow-Up and Treatment of Pulmonary Long COVID. Respiration; international review of thoracic diseases. 2021;100(8):826-41. Available from https://doi.org/10.1159/000517255 | Wrong study design |
| Gaber TAZK, Ashish A, Unsworth A, Martindale J. Are mRNA covid 19 vaccines safe in long covid patients? A health care workers perspective. British Journal of Medical Practitioners 2021;14. Available from bjmp.org/content/are-mrna-covid-19-vaccines-safe-long-covid-patients-health-care-workers-perspective | Wrong study design |
| Gamal Dalia M, Ibrahim Rehab A, Farid Samaan S. Post COVID-19 syndrome in a prospective cohort study of Egyptian patients. Egyptian Rheumatology and Rehabilitation. 2022;49(1):12-. Available from https://doi.org/10.1186/s43166-021-00104-y | Wrong study design |
| Garcia-Molina A, Espina-Bou M, Rodriguez-Rajo P, Sanchez-Carrion R, Ensenat-Cantallops A. Neuropsychological rehabilitation program for patients with post-COVID-19 syndrome: A clinical experience. Programa de rehabilitacion neuropsicologica en pacientes con sindrome post-COVID-19: una experiencia clinica. 2021. Available from https://doi.org/10.1016/j.nrl.2021.03.008 | Wrong study design |
| Geppe NA, Glazachev OS, Timofeev YS, Shakhnazarova MD, Kolosova NG, Samartseva VG, et al. Hypoxic conditioning in comprehensive rehabilitation of children with bronchial asthma after coronavirus infection. Voprosy Prakticheskoi Pediatrii. 2021;16(4):7-15. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-1538972 | Wrong population |
| Gilmutdinova IR, Kolyshenkov VA, Lapickaya KA, Trepova AS, Vasileva VA, Prosvirnin AN, et al. Telemedicine platform COVIDREHAB for remote rehabilitation of patients after COVID-19. Eur J Transl Myol. 2021. Available from https://doi.org/10.4081/ejtm.2021.9783 | Wrong study design |
| Gloeckl R, Leitl D, Jarosch I, Schneeberger T, Christoph. N, Stenzel N, et al. Benefits of pulmonary rehabilitation in COVID-19 – a prospective observational cohort study. ERJ Open Research. 2021:00108-2021. Available from https://doi.org/10.1183/23120541.00108-2021 | Wrong study design |
| Glunčič TJ, Muršič D, Basara L, Vranic L, Močan A, Makek MJ, Samaržija M. Overview of symptoms of ongoing symptomatic and post-COVID-19 patients who were reffered to pulmonary rehabilitation - First single-centre experience in croatia. Psychiatria Danubina 2021;33:565-71. Available from https://www.psychiatria-danubina.com/UserDocsImages/pdf/ dnb_vol33_noSuppl%204/dnb_vol33_noSuppl%204_565.pdf | Wrong population |
| Goel N, Goyal N, Nagaraja R, Kumar R. Systemic corticosteroids for management of 'long-COVID': an evaluation after 3 months of treatment. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace. 2021. Available from https://doi.org/10.4081/monaldi.2021.1981 | Wrong study design |
| Gogoll C, Leo F, Schueller PO, Grohe C. [Post-COVID sequela of the lung - follow up and treatment]. Post-COVID und die Lunge. 2021;146(21):e113. Available from https://doi.org/10.1055/a-1492-8808 | Wrong study design |
| Goodwin VA, Allan L, Bethel A, Cowley A, Cross JL, Day J, et al. Rehabilitation to enable recovery from COVID-19: a rapid systematic review. Physiotherapy. 2021. Available from https://doi.org/10.1016/j.physio.2021.01.007 | Wrong population |
| Gore S, Keysor J. COVID-19 Post-Acute Sequela Rehabilitation: A look to the future through the lens of COPD and Pulmonary Rehabilitation. Archives of rehabilitation research and clinical translation. 2022:100185. Available from https://doi.org/10.1016/j.arrct.2022.100185 | Wrong study design |
| Granger C, Hlal O, Mercier E, Bordart E, Teule L, Colombain L, et al. Description des séquelles à 3 mois d’une COVID grave chez une population jeune et comorbide. Infectious Diseases Now. 2021;51(5):S15-S6. Available from https://doi.org/10.1016/j.idnow.2021.06.006 | Wrong study design |
| Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. The BMJ. 2020;370. Available from https://doi.org/10.1136/bmj.m3026 | Wrong study design |
| Grigoletto I, Cavalheri V, Lima FFd, Ramos EMC. Recovery after COVID-19: The potential role of pulmonary rehabilitation. Brazilian journal of physical therapy. 2020;24(6):463-4. Available from https://doi.org/10.1016/j.bjpt.2020.07.002 | Wrong study design |
| Grissmer J. Acupuncture for COVID Long-Haulers: Pt. 1: 5 Element Acupuncture basis for diagnosis and treatment. Acupuncture Today. 2021;22(8):1-33. Available from https://www.acupuncturetoday.com/digital/index.php?i=762&a_id=34054&pn=2&r=t&Page=1 | Wrong study design |
| Grosbois J-M, Gephine S, Le Rouzic O, Chenivesse C. Feasibility, safety and effectiveness of remote pulmonary rehabilitation during COVID-19 pandemic. Respiratory medicine and research. 2021;80:100846. Available from https://doi.org/10.1016/j.resmer.2021.100846 | Wrong study design |
| Grund S, Bauer J. [Long COVID Syndrome in frail older persons - complex to diagnose and treat]. Long-Covid gefahrdet bei alteren Patienten die Funktionalitat. 2022;164:42-47. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8765814/ | Wrong study design |
| Guck AJ, Buck K, Lehockey K. Psychological complications of COVID-19 following hospitalization and ICU discharge: Recommendations for treatment. Professional Psychology: Research and Practice. 2021. Available from https://doi.org/10.1037/pro0000402 | Wrong study design |
| Gustavson AM, Rud B, Sullivan EK, Beckett A, Gause LR. Role and impact of interdisciplinary rehabilitation in an acute COVID-19 recovery unit. J Am Geriatr Soc. 2021;69(4):878-81. Available from https://doi.org/10.1111/jgs.17060 | Wrong study design |
| Hameed F, Palatulan E, Jaywant A, Said R, Lau C, Sood V, et al. Outcomes of a COVID-19 recovery program for patients hospitalized with SARS-CoV-2 infection in New York City: A prospective cohort study. PM R. 2021. Available from: https://doi.org/10.1002/pmrj.12578 | Wrong population |
| Hameed F, Palatulan E, Jaywant A, Said R, Lau C, Sood V, et al. Reply to letter re "Outcomes of a COVID-19 Recovery Program for Patients Hospitalized with SARS-CoV-2 Infection in New York City: A Prospective Cohort Study.". PM & R : the journal of injury, function, and rehabilitation. 2021. Available from https://doi.org/10.1002/pmrj.12629 | Wrong study design |
| Hanna G, Bankler S, Schandl A, Roel M, Hedman A, Franko MA, et al. The role of ventilatory support for long-term outcomes after critical infection with COVID-19: A prospective cohort study. The clinical respiratory journal. 2021. Available from https://doi.org/10.1111/crj.13453 | Wrong population |
| Haouchan R, Riachy M, Harmouche C, Naoum Z, Salameh M, Merheb P, et al. Late Breaking Abstract - Functional benefits of post COVID-19 multidisciplinary pulmonary rehabilitation program. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.PA3914 | Wrong study design |
| Hayden MC, Limbach MSMMSSGJKNDSK. Effectiveness of a Three-Week Inpatient Pulmonary Rehabilitation Program for Patients after COVID-19: A Prospective Observational Study. Int. J. Environ. Res. Public Health 2021;18:9001-01. Available from https://doi.org/10.3390/ijerph18179001 | Wrong control |
| Hayden MC, Limbach M, Schuler M, Merkl S, Schwarzl G, Jakab K, Nowak D, Schultz K. Short-term Effects of a Three-week Inpatient Post-COVID-19 Pulmonary Rehabilitation Program - a Prospective Observational Study2021 2021. Available from https://doi.org/10.21203/rs.3.rs-578230/v1 | Wrong study design |
| He J, Yang L, Pang J, Dai L, Zhu J, Deng Y, et al. Efficacy of simplified-cognitive behavioral therapy for insomnia(S-CBTI) among female COVID-19 patients with insomnia symptom in Wuhan mobile cabin hospital. Sleep & breathing = Schlaf & Atmung. 2021;25(4):2213-9. Available from https://doi.org/10.1007/s11325-021-02350-y | Wrong study design |
| Heald A, Riste L, Walther A, Stedman M, Mukherjee A, Perrin R. Reducing fatigue-related symptoms in Long COVID-19: finding an intervention that works. BJPsych Open. 2021;7:S254-S5. Available from https://doi.org/10.1192/bjo.2021.681 | Wrong study design |
| Heald A, Perrin R, Walther A, Stedman M, Hann M, Mukherjee A, et al. Reducing fatigue-related symptoms in Long COVID-19: a preliminary report of a lymphatic drainage intervention. Cardiovascular endocrinology & metabolism. 2022;11(2):e0261. Available from https://doi.org/10.1097/XCE.0000000000000261 | Wrong study design |
| Hennigs JK, Oqueka T, Harbaum L, Klose H. [Organ-specific sequelae of COVID-19 in adults]. Organbezogene Folgeerscheinungen von COVID-19 bei Erwachsenen. 2022. Available from https://doi.org/10.1007/s00103-022-03513-2 | Wrong study design |
| Herman B, Viwattanakulvanid P, Dzulhadj A, Oo AC, Patricia K, Pongpanich S. EFFECT OF FULL VACCINATION AND POST-COVID OLFACTORY DYSFUNCTION IN RECOVERED COVID-19 PATIENT. A RETROSPECTIVE LONGITUDINAL STUDY WITH PROPENSITY MATCHING 2022. Available from https://doi.org/10.1101/2022.01.10.22269007 | Wrong study design |
| Holtslag HR, van den Borst B, Reijers MHE, Dettling DS. Post-COVID-19 rehabilitation; a matter of customisation. Nazorg voor covid-19-patienten. 2020;164. | Wrong study design |
| Howard-Jones AR, Burgner DP, Crawford NW, Goeman E, Gray PE, Hsu P, et al. COVID-19 in children. II: Pathogenesis, disease spectrum and management. J Paediatr Child Health. 2021. Available from https://doi.org/10.1111/jpc.15811 | Wrong study design |
| Huffman S, Badran B, Dancy M, Austelle C, Kautz S, George M. At-Home Telemedicine Controlled taVNS Twice Daily for 4 weeks is Feasible and Safe for Long COVID Symptoms. Brain Stimul. 2021;14(6):1702-3. Available from https://doi.org/10.1016/j.brs.2021.10.367 | Wrong study design |
| hussien M, Hussien A, ismail W, alsoubky M, ramzy S, Shahin M. Efficacy of pentasodium diethylenetriamine pentaacetate in ameliorating anosmia post COVD-19 (preprint)2022 2022. Available from https://doi.org/10.22541/au.164607067.70886700/v1 | Wrong study design |
| Hussain A, Khurana A, Kumar G, Abhishek S, Raj K. Pulmonary rehabilitation in Covid pneumonia sequelae: so near yet so far. ERJ Open Research. 2021:00398-2021. Available from https://doi.org/10.1183/23120541.00398-2021 | Wrong study design |
| Hylton H, Pfeffer PE, Robson C, Goodfellow H, Murray E, Ricketts W. Rapid design and implementation of a personalised holistic post-COVID recovery and rehab app. Thorax. 2021;76:A236. Available from http://dx.doi.org/10.1136/thorax-2020-BTSabstracts.412 | Wrong study design |
| Imamura M, Mirisola AR, Ribeiro FdQ, De Pretto LR, Alfieri FM, Delgado VR, et al. Rehabilitation of patients after COVID-19 recovery: An experience at the Physical and Rehabilitation Medicine Institute and Lucy Montoro Rehabilitation Institute. Clinics (Sao Paulo, Brazil). 2021;76:e2804. Available from https://doi.org/10.6061/clinics/2021/e2804 | Wrong study design |
| Jadhav K, Jariwala P. ‘Ivabradin’ versus ‘Carvedilol’ in the management of Post-COVID-19 palpitation with sinus tachycardia. Indian Heart J. 2020;72:S33. Available from https://doi.org/10.1016/j.ihj.2020.11.092 | Wrong study design |
| Jadhav K, Jariwala P. Ivabradine versus carvedilol in the management of palpitation with sinus tachycardia among recovered COVID-19 patients. J Cardiol Cardiovasc Med. 2020;5:176-80. Available from https://doi.org/10.29328/journal.jccm.1001107 | Wrong population |
| Jafar A, Lasso A, Shorr R, Hutton B, Kilty S. Olfactory recovery following infection with COVID-19: A systematic review. PLoS One. 2021;16(11):e0259321. Available from https://doi.org/10.1371/journal.pone.0259321 | Wrong intervention |
| Jain E, Harmon EY, Sonagere MB. Functional Outcomes and Post-Discharge Care Sought by Patients with COVID-19 Compared to Matched Controls After Completing Inpatient Acute Rehabilitation. PM & R : the journal of injury, function, and rehabilitation. 2021. Available from https://doi.org/10.1002/pmrj.12607 | Wrong population |
| Jalalizadeh M, Buosi K, Dionato FAV, Dal Col LSB, Giacomelli CF, Ferrari KL, et al. Randomized clinical trial of BCG vaccine in patients with convalescent COVID-19: Clinical evolution, adverse events, and humoral immune response. J Intern Med. 2022. Available from https://doi.org/10.1111/joim.13523 | Wrong population |
| Jalusic Gluncic T, Mursic D, Basara L, Vranic L, Mocan A, Jankovic Makek M, et al. Overview of Symptoms of Ongoing Symptomatic and Post-COVID-19 Patients Who Were Reffered to Pulmonary Rehabilitation - First Single-Centre Experience in Croatia. Psychiatria Danubina. 2021;33:565-71. Available from https://pubmed.ncbi.nlm.nih.gov/34718282/ | Wrong study design |
| Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947. Available from https://doi.org/10.1016/j.ejphar.2021.173947 | Wrong population |
| Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, Martínez-Cava A, Franco-López F, Sánchez-Alcaraz Martínez BJ, et al. Post-covid-19 syndrome and the potential benefits of exercise. Int J Environ Res Public Health. 2021;18(10). Available from https://doi.org/10.3390/ijerph18105329 | Wrong study design |
| Johansson J, Levi R, Jakobsson M, Gunnarsson S, Samuelsson K. Multiprofessional Neurorehabilitation After COVID-19 Infection Should Include Assessment of Visual Function. Arch Rehabil Res Clin Transl. 2022;4(2):100184. Available from https://doi.org/10.1016/j.arrct.2022.100184 | Wrong study design |
| Kabalkin Y, Gil M, Lifshitz E, Moav A, Kabessa M, Jaber S, et al. Effects of SARS-Corona virus 2 on IVF treatment parameters: A cohort study of post COVID-19 patients. Hum. Reprod. 2021;36:130-30. | Wrong study design |
| Kader M, Hossain MA, Reddy V, Perera NKP, Rashid M. Effects of short-term breathing exercises on respiratory recovery in patients with COVID-19: a quasi-experimental study. BMC Sports Science, Medicine and Rehabilitation. 2022;14(1). Available from https://doi.org/10.1186/s13102-022-00451-z | Wrong population |
| Kandakurti PK, Amaravadi SK. Management and Rehabilitation of COVID-19: A Physiotherapist Perspective. Critical Reviews in Physical and Rehabilitation Medicine. 2021;33(1):1-15. Available from https://doi.org/10.1615/CritRevPhysRehabilMed.2021037383 | Wrong study design |
| Kardes S. Spa therapy (balneotherapy) for rehabilitation of survivors of COVID-19 with persistent symptoms. Med Hypotheses. 2021;146:110472. Available from https://www.sciencedirect.com/science/article/ pii/S0306987720333636?via%3Dihub | Wrong study design |
| Karime C, Doulaye Seydou M, Ragland J, Wyrick B, Ijaz M, Khan AM. Pulmonary Function at 1- and 2.5-Months Following Hospital Discharge in Patients with Coronavirus Disease 2019. A Preliminary Study Investigating the Effect of Albuterol Sulfate with or Without Inhaled Corticosteroids. Am J Respir Crit Care Med. 2021;203(9). Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1277768 | Wrong study design |
| Karosanidze I, Kiladze U, Kirtadze N, Giorgadze M, Amashukeli N, Parulava N, et al. Efficacy of Adaptogens in Patients with Long COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals (Basel). 2022;15(3). Available from https://doi.org/10.3390/ph15030345 | Wrong population |
| Karthikeyan T. Therapeutic effectiveness of diaphragmatic with costal breathing exercises on C-19 PEFR patients. Intensive Care Medicine Experimental. 2021;9 | Wrong study design |
| Kasnakova P, Kilova K. Recovery and rehabilitation of patients with COVID-19 and post-COVID-19 syndrome. Kuwait Med. J. 2021;53:346-47. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-1431496 | Wrong study design |
| Kazama I. Stabilizing mast cells by commonly used drugs: a novel therapeutic target to relieve post-COVID syndrome? Drug Discov Ther. 2020;14(5):259-61. Available from https://doi.org/10.5582/ddt.2020.03095 | Wrong study design |
| Khunti K, Davies MJ, Kosiborod MN, Nauck MA. Long COVID – metabolic risk factors and novel therapeutic management. Nature Reviews Endocrinology. 2021. Available from https://doi.org/10.1038/s41574-021-00495-0 | Wrong study design |
| Kiekens C, Boldrini P, Andreoli A, Avesani R, Gamna F, Grandi M, et al. Rehabilitation and respiratory management in the acute and early post-acute phase "instant paper from the field" on rehabilitation answers to the COVID-19 emergency. Eur J Phys Rehabil Med. 2020;56(3):323-6. Available from https://doi.org/10.23736/s1973-9087.20.06305-4 | Wrong study design |
| King M, Byrne A, Denehy L, Graham P, Douglas B, de Toni P, et al. Feasibility of a Group-Based Telerehabilitation Intervention for Long COVID Management (preprint) 2022. Available from https://doi.org/10.21203/rs.3.rs-1452186/v1 | Wrong study design |
| Kireyev IV, Zhabotynska NV, Bakumenko MG, Khyzhnyak VM, Knizhenko IB. Rehabilitation in Post COVID-19 Neurological Syndrome. Acta Balneol. 2022;64(1):11-5. Available from https://doi.org/10.36740/ABal202201102 | Wrong population |
| Kirkner RM. Steroids reduced COVID-19 persistent lung dysfunction. Chest Physician. 2021;16(4):10-. | Wrong study design |
| Knight F, Cornish L, Shen X, Thomas C. Is Pulmonary Rehabilitation (PR) effective in people recovering from severe COVID-19 (C-19) pneumonia? Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.PA2264 | Wrong study design |
| Kokhan S, Romanova E, Nadeina L, Baatar B, Shagdarsuren O, Purevdorj D. EFFECT OF PHYSICAL REHABILATION ON THE FUNCTIONAL STATE OF POST COVID-19 PATIENTS. Laplage Em Revista. 2021;7(3):675-81. Available from https://doi.org/10.24115/S2446-6220202173A1475p.675-681 | Wrong study design |
| Kokhan S, Vlasava S, Kolokoltsev M, Bayankin O, Kispayev T, Trofimova N, et al. Postcovid physical rehabilitation at the sanatorium. Journal of Physical Education and Sport. 2022;22(3):607-13. Available from https://www.efsupit.ro/images/stories/martie2022/Art%2076.pdf | Wrong study design |
| Kolditz M, Beyer-Westendorf J, von Bonin S, Koschel DS. [Persistent dyspnea after COVID-19: Suggestions for follow-up care]. Persistierende Dyspnoe nach COVID-19: Vorschlage zur hausarztlichen Nachsorge. 2021;163(8):52-5. Available from https://doi.org/10.1007/s15006-021-9842-6 | Wrong study design |
| Kryvenko VI, Kolesnyk MY, Bielenichev IF, Pavlov SV. Thiotriazolin effectiveness in complex treatment of patients with post-COVID syndrome. Zaporozhye Medical Journal. 2021;23(3):402-11. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1315014 | Wrong population |
| Kumar Khurana A, Hussain A, Goyal A, Karna ST, Saigal S, Krishnan Soman R, et al. Six-Week Hospital-Based Pulmonary Rehabilitation in Covid Pneumonia ICU Survivors: Experience from a Tertiary Care Center in Central India. Turkish thoracic journal. 2022;23(2):89-96. Available from https://doi.org/10.5152/TurkThoracJ.2022.21159 | Wrong population |
| Kwiatkowska K, Partyka O, Pajewska M, Czerw A. POST COVID-19 PATIENTS' REHABILITATION - POTENTIAL OF USING HALOTHERAPY IN THE FORM OF GENERALLYACCESSIBLE INHALATORIA WITH DRY SALT AEROSOL. Acta Pol Pharm. 2021;78(6):749-54. Available from https://doi.org/10.32383/appdr/146494 | Wrong study design |
| LaFond E, Weidman K, Lief L. Care of the postcoronavirus disease 2019 patient. Current opinion in pulmonary medicine, 2021;27(3):199-204. Available from https://doi.org/10.1097/MCP.0000000000000767 | Wrong intervention |
| Laine C, Cotton D. COVID-19: Evaluation and Care of Patients With Persistent Symptoms Following Acute SARS-CoV-2 Infection. Ann. Intern. Med. 2021;174:1159-60. Available from https://doi.org/10.7326/M21-2342 | Wrong study design |
| Lal, A., Mishra, A. K., John, K., & Akhtar, J. Corticosteroids and rehabilitation in COVID-19 survivors. Journal of the Formosan Medical Association = Taiwan yi zhi, 2021;120(5): 1284-85. Available from https://doi.org/10.1016/j.jfma.2020.12.005 | Wrong intervention |
| Larinskiy N, Larinskaya I, Byalovskiy Y, Glotov S, Shakhanov A. Evaluation of the effectiveness of low-frequency magnetotherapy in the rehabilitation of patients with pneumonia caused by the SARS-CoV-2 virus (the causative agent of COVID-19). Pakistan Journal of Medical and Health Sciences. 2021;15(6):1706-8. Available from https://doi.org/10.53350/pjmhs211561706 | Wrong population |
| Lasa JJ, Alali A, Anders M, Tume SC, Muscal E, Tejtel SKS, et al. Cardiovascular sequelae from COVID-19: perspectives from a paediatric cardiac ICU. Cardiol Young. 2022:1-8. Available from https://doi.org/10.1017/S1047951122000130 | Wrong study design |
| Lassen MCH, Skaarup KG, Lind JN, Alhakak AS, Sengeløv M, Nielsen AB, et al. Recovery of cardiac function following COVID‐19 – ECHOVID‐19: a prospective longitudinal cohort study. Eur. J. Heart Fail. 2021;23:1903-12. Available from https://doi.org/10.1002/ejhf.2347 | Wrong study design |
| Law S, Leung AW, Xu C. Tai-Chi and Baduanjin during treatment and rehabilitation of older adults with COVID-19. Asian Journal of Gerontology and Geriatrics 2020;15:96-96. Available from https://doi.org/10.12809/ajgg-2020-435-letter | Wrong study design |
| Lazzeri M, Lanza A, Bellini R, Bellofiore A, Cecchetto S, Colombo A, et al. Respiratory physiotherapy in patients with COVID-19 infection in acute setting: A Position Paper of the Italian Association of Respiratory Physiotherapists (ARIR). Monaldi Archives for Chest Disease. 2020;90(1):163-8. Available from https://doi.org/10.4081/monaldi.2020.1285 | Wrong study design |
| Leckie T, Hunter A, Hardy B, Palmer A, Standing M-K, Stoner G, et al. A socially distanced and digitally enhanced COVID-19 rehabilitation programme. Clin Med. 2021;21:57. Available from https://doi.org/10.7861/clinmed.21-2-s57 | Wrong study design |
| Lee KM, Ko HJ, Lee GH, Kim AS, Lee DW. A well‐structured follow‐up program is required after recovery from coronavirus disease 2019 (Covid‐19); release from quarantine is not the end of treatment. Journal of Clinical Medicine. 2021;10(11). Available from https://doi.org/10.3390/jcm10112329 | Wrong study design |
| Lee-Mateus AY, Hernandez-Rojas D, Castillo-Larios R, Walsh K, Abia-Trujillo D, Fernandez-Bussy S. Organizing pneumonia post COVID-19: Outcomes of treatment with corticosteroids in the outpatient setting. Respirology 2021;26:176. Available from https://doi.org/10.1111/resp.14150_258 | Wrong study design |
| Leeb S. Long-Covid — Effiziente Behandlungs- Strategien Mit Akupunktur und Homöopathischer Unterstützung Long-COVID — Efficient Acupuncture Strategies Supported by Homeopathic Treatment. Akupunktur & Aurikulomedizin 2021;47:32-35. Available from https://doi.org/10.1007/s15009-021-5749-7 | Wrong study design |
| Lei J, Yang L, Wen G, Qumu S, Ren X, Yang T. Pulmonary telerehabilitation and efficacy among discharged COVID-19 patients: Rational and design of a prospective real-world study. The clinical respiratory journal 2021. Available from https://doi.org/10.1111/crj.13422 | Wrong study design |
| Leite VF, Rampim DB, Jorge VC, de Lima MdCC, Cezarino LG, da Rocha CN, et al. Persistent Symptoms and Disability After COVID-19 Hospitalization: Data From a Comprehensive Telerehabilitation Program. Arch Phys Med Rehabil. 2021;102(7):1308-16. Available from https://doi.org/10.1016/j.apmr.2021.03.001 | Wrong study design |
| Leitl D, Schneeberger T, Glöckl R, Jarosch I, Rembert Koczulla A. Rehabilitation bei Post-COVID-19-Patienten - individuell und zielgerichtet. Pneumo News. 2022;14(1):30-9. Available from https://doi.org/10.1007/s15033-022-2806-4 | Wrong study design |
| Li L, An X, Zhang Q, Tao J, He J, Chen Y, et al. Shumian capsule (舒眠胶囊) improves symptoms of sleep mood disorder in convalescent patients of Corona Virus Disease 2019. J Tradit Chin Med. 2021;41(6):974-81. Available from https://doi.org/10.19852/j.cnki.jtcm.2021.06.015 | Obvious inconsistencies in data |
| Li L, Gou CY, Li XM, Song WY, Wang XJ, Li HY, et al. Effects of Chinese Medicine on Symptoms, Syndrome Evolution, and Lung Inflammation Absorption in COVID-19 Convalescent Patients during 84-Day Follow-up after Hospital Discharge: A Prospective Cohort and Nested Case-Control Study. Chin J Integr Med. 2021;27(4):245-51. Available from https://doi.org/10.1007/s11655-021-3328-3 | Wrong population |
| Lim L. Treating COVID-19 with photobiomodulation-short-term recovery and long-haul neuroregulation. NeuroRegulation. 2021;8(4):207-8. | Wrong study design |
| Lima Bosi P, de Freitas Januzzi LF, Barreto de Paula P, Carvalho de Oliveira C, Scianni CA, Nunes da Costa TA, et al. A importância da reabilitação pulmonar em pacientes com COVID-19. Fisioterapia Brasil. 2021;22(2):261-71. Available from https://doi.org/10.33233/fb.v22i2.4288 | Wrong study design |
| Limbach M, Hayden M, Nowak D, Schwarzl G, Jakab K, Merkl S, et al. Pneumological Rehabilitation in Post-Covid-19 Patients: Experiences and Short-term Treatment Results. Pneumologie 2021;75:S51-S51. | Wrong study design |
| Lin Y, Saper R, Patil SJ. Long COVID Shared Medical Appointments: Lifestyle and Mind-Body Medicine With Peer Support. Ann Fam Med. 2022. Available from https://doi.org/10.7302/3956 | Wrong study design |
| Liska D, Andreansky M. Rehabilitation and physical activity for COVID-19 patients in the post infection period. Bratislava Medical Journal. 2021;122(5):310-4. Available from https://doi.org/10.4149/BLL_2021_052 | Wrong study design |
| Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement Ther Clin Pract. 2020 May;39:101166. Available from https://doi.org/10.1016/j.ctcp.2020.101166 | Wrong population |
| Liu Y, Yang YQ, Liu Y, Pei SL, Yang HH, Wu JJ, et al. Effects of group psychological intervention combined with pulmonary rehabilitation exercises on anxiety and sleep disorders in patients with mild coronavirus disease 2019 (COVID-19) infections in a Fangcang hospital. Psychol Health Med. 2021:1-11. Available from: https://doi.org/10.1080/13548506.2021.1916956 | Wrong population |
| Lu ZH, Yang CL, Yang GG, Pan WX, Tian LG, Zheng JX, et al. Efficacy of the combination of modern medicine and traditional Chinese medicine in pulmonary fibrosis arising as a sequelae in convalescent COVID-19 patients: a randomized multicenter trial. Infectious diseases of poverty. 2021;10(1):31. Available from https://doi.org/10.1186/s40249-021-00813-8 | Wrong study design |
| Lucidi D, Molinari G, Silvestri M, De Corso E, Guaraldi G, Mussini C, et al. Patient-reported olfactory recovery after SARS-CoV-2 infection: A 6-month follow-up study. International forum of allergy & rhinology. 2021. Available from https://doi.org/10.1002/alr.22775 | Wrong intervention |
| Ludvigsson JF. Spanish telemedicine data on 8 children support concept of "long covid" in children. Acta Paediatr. 2021. Available from https://doi.org/10.1111/apa.15869 | Wrong study design |
| Lyadov KV, Koneva ES, Polushkin VG, Sultanov E, Lukashin MA. Randomized controlled study on pulmonary rehabilitation in COVID-19 patients with pneumonia. Pulmonologiya. 2020;30(5):569‐76. Available from https://doi.org/10.18093/0869-0189-2020-30-5-569-576 | Wrong population |
| Malcolm MP. Occupational Therapy in Postacute Care for Survivors of COVID-19: Research Gaps We Need to Fill. Am. J. Occup. Ther. 2021;75:1-5. Available from https://doi.org/10.5014/ajot.2021.049195 | Wrong study design |
| Maldonado-Belmonte MJ, Fernández-Jiménez E, Sánchez-Polo MT. On verbal working memory. Descriptive study in post-intensive care syndrome patients after COVID-19 infection in a functional rehabilitation unit in Spain. A pilot study. Eur. Psychiatry 2021;64:S664-S65. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1357364 | Wrong study design |
| Maldonado-Belmonte MJ, Fernández-Jiménez E, Román-Belmonte JM. On general cognitive functioning. Descriptive study in post-intensive care syndrome patients after COVID-19 infection in a functional rehabilitation unit in Spain. A pilot study. Eur. Psychiatry 2021;64:S665-S65. Available from https://doi.org/10.1192/j.eurpsy.2021.1765 | Wrong study design |
| Maldonado-Belmonte MJ, Fernández-Jiménez E, Vázquez-Sasot A. On delayed verbal learning. Descriptive study in post-intensive care syndrome patients after COVID-19 infection in a functional rehabilitation unit in Spain. A pilot study. Eur. Psychiatry 2021;64:S664-S64. Available from https://doi.org/10.1192/j.eurpsy.2021.1763 | Wrong study design |
| Maltser S, Trovato E, Fusco HN, Sison CP, Ambrose AF, Herrera J, et al. Challenges and Lessons Learned for Acute Inpatient Rehabilitation of Persons With COVID-19: Clinical Presentation, Assessment, Needs, and Services Utilization. Am J Phys Med Rehabil. 2021;100(12):1115-23. Available from https://doi.org/10.1097/PHM.0000000000001887 | Wrong study design |
| Malyavin AG, Babak SL, Gorbunova MV. Respiratory rehabilitation for post-COVID-19 patients. Russian Archives of Internal Medicine, 2021;11(1):22-33. Available from https://doi.org/10.20514/2226-6704-2021-11-1-22-33 | Wrong study design |
| Maniscalco M, Ambrosino P, Fuschillo S, Stufano S, Sanduzzi A, Matera MG, et al. Bronchodilator reversibility testing in post-COVID-19 patients undergoing pulmonary rehabilitation. Respir Med. 2021;182:106401. Available from https://doi.org/10.1016/j.rmed.2021.106401 | Wrong study design |
| Maniscalco M, Fuschillo S, Ambrosino P, Martucci M, Papa A, Matera MG, et al. Preexisting cardiorespiratory comorbidity does not preclude the success of multidisciplinary rehabilitation in post-COVID-19 patients. Respir Med. 2021;184:106470. Available from https://doi.org/10.1016/j.rmed.2021.106470 | Wrong study design |
| Marin T, Maxel X, Robin A, Stubbe L. Evidence-based assessment of potential therapeutic effects of adjunct osteopathic medicine for multidisciplinary care of acute and convalescent COVID-19 patients. Explore (New York, NY). 2021;17(2):141-7. Available from https://doi.org/10.1016/j.explore.2020.09.006 | Wrong study design |
| Mazza MG, Palladini M, Zanardi R, Benedetti F. P.0404 Rapid antidepressant response to first-line selective serotonin reuptake inhibitors in post-COVID-19 depression. Eur. Neuropsychopharmacol. 2021;53:S292-S93. Available from https://doi.org/10.1016/j.euroneuro.2021.09.009 | Wrong study design |
| McGregor G, Sandhu H, Bruce J, Sheehan B, McWilliams D, Yeung J, et al. Rehabilitation Exercise and psycholoGical support After covid-19 InfectioN' (REGAIN): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22(1):8. Available from https://doi.org/10.1186/s13063-020-04978-9 | Wrong study design |
| McNarry M, Shelley J, Hudson J, Saynor Z, Duckers J, Lewis K, et al. Late Breaking Abstract - A randomised control trial using inspiratory muscle training in post-COVID-19 rehabilitation. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.OA169 | Wrong study design |
| Melegari G, Giuliani E, Dallai C, Veronesi L, Bertellini E, Osmenaj S, et al. Intensive Care Patients from the First COVID-19 Wave: One-Year Survival after Tocilizumab Treatment. Journal of personalized medicine. 2021;11(11). Available from https://doi.org/10.3390/jpm11111234 | Wrong population |
| Membrilla JA, Caronna E, Trigo-López J, González-Martínez A, Layos-Romero A, Pozo-Rosich P, et al. Persistent headache after COVID-19: Pathophysioloy, clinic and treatment. Neurology Perspectives 2021;1:S31-S36. Available from https://doi.org/10.1016/j.neurop.2021.10.003 | Wrong study design |
| Michaud S. Evolving Approaches to Testing and Treatment for LONG COVID. Clinical Laboratory News. 2021;47(9):10-4. Available from https://www.aacc.org/cln/articles/2021/november/evolving-approaches-to-testing-and-treatment-for-long-covid | Wrong study design |
| Milne A, Maskell S, Sharp C, Hamilton FW, Arnold DT. Impact of dexamethasone on persistent symptoms of COVID-19: an observational study (preprint). 2021. Available from https://doi.org/10.1101/2021.11.17.21266392 | Wrong population |
| Missé RG et al. Transcranial direct current electrical stimulation in combination with aerobic exercise is effective in reducing fatigue and pain in post-COVID-19 systemic autoimmune rheumatic patients (preprint) 2021. Available from https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/ppcovidwho-291857 | Wrong study design |
| Mitrani MI, Bellio MA, Meglin A, Khan A, Xu X, Haskell G, et al. Treatment of a COVID-19 long hauler with an amniotic fluid-derived extracellular vesicle biologic. Respiratory Medicine Case Reports 2021:101502-02. Available from https://doi.org/10.1016/j.rmcr.2021.101502 | Wrong study design |
| Modi P, Kulkarni S, Nair G, Kapur R, Chaudhary S, Langade D, et al. Evaluation of post-COVID functional capacity and oxygen desaturation using 6-minute walk test- An observational study. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.PA3162 | Wrong study design |
| Mohr A, Dannerbeck L, Lange TJ, Pfeifer M, Blaas S, Salzberger B, et al. Cardiopulmonary exercise pattern in patients with persistent dyspnoea after recovery from COVID-19. Multidisciplinary respiratory medicine. 2021;16(1):732. Available from https://doi.org/10.4081/mrm.2021.732 | Wrong intervention |
| Moretta P, Maniscalco M, Papa A, Lanzillo A, Trojano L, Ambrosino P. Cognitive impairment and endothelial dysfunction in convalescent COVID-19 patients undergoing rehabilitation. Eur. J. Clin. Invest. 2022;52. Available from https://doi.org/10.1111/eci.13726 | Wrong study design |
| Morrow G. État des connaissances - Organisation des soins et des services pour la prévention et la prise en charge des affections post-COVID-19 2022 | Wrong study design |
| Mu M. Effect on Novel Corona-Virus Pneumonia Patients' Rehabilitation Training of Tibetan Folk Music. Basic Clin Pharmacol Toxicol. 2020;127:267‐. | Wrong study design |
| Myall KJ, Mukherjee B, Castanheira AM, Lam JL, Benedetti G, Mak SM, et al. Persistent Post-COVID-19 Interstitial Lung Disease. An Observational Study of Corticosteroid Treatment. Annals of the American Thoracic Society. 2021;18(5):799-806. Available from https://doi.org/10.1513/AnnalsATS.202008-1002OC | Wrong study design |
| Naeije R, Caravita S. Phenotyping long COVID. Eur. Respir. J. 2021;58. Available from https://doi.org/10.1183/13993003.01763-2021 | Wrong study design |
| Nambi G, Abdelbasset WK, Alrawaili SM, Elsayed SH, Verma A, Vellaiyan A, et al. Comparative effectiveness study of low versus high-intensity aerobic training with resistance training in community-dwelling older men with post-COVID 19 sarcopenia: A randomized controlled trial. Clin Rehabil. 2021:2692155211036956. Available from https://doi.org/10.1177/02692155211036956 | Wrong population |
| Naoi Shunsuke, Nakazato Shunsuke, Kamesako Junya, Sekine Shusuke, Imaizumi Hitoshi. Effect of Respiratory Rehabilitation for a Patient with Severe Pneumonia and Intensive Care Unit Acquired Weakness (ICU-AW) Due to COVID-19. Rigakuryoho Kagaku. 2021;36(5):747-52 | Wrong study design |
| Natarajan A, Shetty A, Delanerolle G, Zeng Y, Zhang Y, Raymont V, et al. A systematic review and meta-analysis of Long COVID symptoms. P. Phiri, Southern Health NHS Foundation Trust Research, Innovation Department Clinical Trials Facility, Moorgreen Hospital, Southampton, United Kingdom 2022. Available from https://doi.org/10.1101/2022.03.08.22272091 | No intervention |
| Naureen Z, Dautaj A, Nodari S, Fioretti F, Dhuli K, Anpilogov K, et al. Proposal of a food supplement for the management of post-COVID syndrome. Eur Rev Med Pharmacol Sci. 2021;25(1):67-73. Available from https://doi.org/10.26355/eurrev_202112_27335 | Wrong control |
| Nazir A, Hasri I. Pathophysiology and rehabilitation management of exercise intolerance in COVID-19 patients. Ann Thorac Med. 2022;17(2):87-93. Available from https://doi.org/10.4103/atm.atm_357_21 | Wrong study design |
| Nct. COVID-19 Long-Haulers Study. Available from https://clinicaltrials.gov/show/NCT04678830 2020 | Wrong study design |
| Nct. Anhydrous Enol-Oxaloacetate (AEO) on Improving Fatigue in Post-COVID-19 Survivors. Available from https://clinicaltrials.gov/show/NCT04592354 2020 | Wrong study design |
| Nct. Symptom-based Rehabilitation Compared to Usual Care in Post-COVID - a Randomized Controlled Trial. https://clinicaltrials.gov/show/NCT05172206 2021. Eur. J. Clin. Invest. 2022;52. Available from https://clinicaltrials.gov/ct2/show/NCT05172206 | Wrong study design |
| Nct. Anosmia and Covid-19. 2022. Available from https://clinicaltrials.gov/ct2/show/NCT05246059 | Wrong study design |
| Negm AM, Salopek A, Zaide M, Meng VJ, Prada C, Chang Y, et al. Rehabilitation at the Time of Pandemic: Patient Journey Recommendations. Front Aging Neurosci. 2022;14:781226. Available from https://doi.org/10.3389/fnagi.2022.781226 | Wrong study design |
| Negrini S, Mills J-A, Arienti C, Kiekens C, Cieza A. "Rehabilitation Research Framework for Patients With COVID-19" Defined by Cochrane Rehabilitation and the World Health Organization Rehabilitation Programme. Arch Phys Med Rehabil. 2021;102(7):1424-30. Available from https://doi.org/10.1016/j.apmr.2021.02.018 | Wrong population |
| Negrini F, de Sire A, Andrenelli E, Lazzarini SG, Patrini M, Ceravolo MG. Rehabilitation and COVID-19: update of the rapid living systematic review by Cochrane Rehabilitation Field as of April 30, 2021. Eur J Phys Rehabil Med. 2021;57(4):663-7. Available from https://doi.org/10.23736/S1973-9087.21.07125-2 | Wrong study design |
| Nesina IA, Golovko EA, Shakula AV, Figurenko NN, Zhilina IG, Khomchenko TN, et al. experience of outpatient rehabilitation of Patients after Pneumonia associated with the New coronavirus Infection coVID-19. Vestnik Vosstanovitel'noj Mediciny. 2021;20(5):4-11. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1614634 | Wrong population |
| Nguyen-Hoang A. Nutrition therapy for long COVID. British journal of nursing (Mark Allen Publishing). 2021;30(21):S28-S9. Available from https://doi.org/10.12968/bjon.2021.30.21.S28 | Wrong study design |
| Njoku IO, Aggarwal A, Bamgartner M, Lever JEP, Fleming TK. Letter regarding "Outcomes of a COVID‐19 recovery program for patients hospitalized with SARS‐CoV‐2 infection in New York City: A prospective cohort study". PM R. 2021;13(8):925-6. Available from https://doi.org/10.1002/pmrj.12653 | Wrong study design |
| Nopp S, Moik F, Klok FA, Gattinger D, Petrovic M, Vonbank K, et al. Late Breaking Abstract - Outpatient pulmonary rehabilitation in patients with long COVID. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.PA2119 | Wrong study design |
| Nopp S, Moik F, Klok FA, Gattinger D, Petrovic M, Vonbank K, et al. Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. Respiration; international review of thoracic diseases. 2022:1-9. Available from https://doi.org/10.1159/000522118 | Wrong study design |
| Nourian R, Niyazi S, Nazarieh M, Sharafi SE, Shahi MHP. IASEM-TUMS COVID-19 Virtual Pulmonary Rehabilitation Framework; Exercise Prescription for Recovered COVID-19 Patients. Asian J Sports Med. 2020;11(4):1-4. Available from https://doi.org/10.5812/asjsm.107575 | Wrong study design |
| Novak P, Cunder K, Petrovic O, Oblak T, Dular K, Zupanc A, et al. Rehabilitation of COVID-19 patients with respiratory failure and critical illness disease in Slovenia: an observational study. International journal of rehabilitation research. Internationale Zeitschrift fur Rehabilitationsforschung. Revue internationale de recherches de readaptation 2022. Available from https://doi.org/10.1097/MRR.0000000000000513 | Wrong study design |
| O'Brien H, Tracey MJ, Ottewill C, O'Brien ME, Morgan RK, Costello RW, et al. An integrated multidisciplinary model of COVID-19 recovery care. Ir J Med Sci. 2021;190(2):461-8. Available from https://doi.org/10.1007/s11845-020-02354-9 | Wrong study design |
| O'Byrne L, Webster KE, MacKeith S, Philpott C, Hopkins C, Burton MJ. Interventions for the treatment of persistent post-COVID-19 olfactory dysfunction. The Cochrane database of systematic reviews. 2021;7:CD013876. Available from https://doi.org/10.1002/14651858.CD013876.pub2 | Wrong study design |
| O´Grady M, Bowen B, Sadlier C, Plant BJ, Kennedy M, Henry MT, et al. An overview of the establishment and delivery of a Virtual Pulmonary Rehabilitation Programme in Cork University Hospital for patients following COVID 19 infection. Ir J Med Sci. 2021;190:S12-S3. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7788179/ | Wrong study design |
| O`Reilly M, Gillen C, Meehan C, Counihan I, Hassan T. Pulmonary rehabilitation programme: A transcendence during COVID-19 pandemic. Ir. Med. J. 2020;113:1-2. Available from https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/en/covidwho-829391 | Wrong study design |
| O'Sullivan O, Barker-Davies RM, Thompson K, Bahadur S, Gough M, Lewis S, et al. Rehabilitation post-COVID-19: cross-sectional observations using the Stanford Hall remote assessment tool. BMJ military health. 2021. Available from https://doi.org/10.1136/bmjmilitary-2021-001856 | Wrong intervention |
| Ocal S. Pulmonary rehabilitation. J Crit Intensive Care. 2020;11:16-7. Available from https://doi.org/10.37678/dcybd.2020.2367 | Wrong study design |
| Ono R, Arita R, Takayama S, Kikuchi A, Ohsawa M, Saito N, et al. Kampo Medicine Promotes Early Recovery From Coronavirus Disease 2019-Related Olfactory Dysfunction: A Retrospective Observational Study. Front Pharmacol. 2022;13:844072. Available from https://doi.org/10.3389/fphar.2022.844072 | Wrong population |
| Ostojic SM. Can creatine help in pulmonary rehabilitation after COVID-19? Ther Adv Respir Dis. 2020;14:1753466620971144. Available from https://doi.org/10.1177/1753466620971144 | Wrong study design |
| Oudjedi A. The Potential Benefits of antihistamine therapy and exercise rehabilitation in women with Post-COVID-19 Syndrome. Apunts Sports Medicine. 2022:100384-. Available from https://doi.org/10.1016/j.apunsm.2022.100384 | Wrong study design |
| Ouellette NH, Bellinger L, Leonard J. Examining the Effectiveness of OT in the Treatment of Patients Recovering From COVID-19 in the Rehabilitation Setting. Am. J. Occup. Ther. 2021;75:1-1. Available from https://doi.org/10.5014/ajot.2021.75S2-RP157 | Wrong study design |
| Pal GK, Nanda N, Renugasundari M, Pal P, Pachegaonkar U. Acute effects of prone asanas and pal’s pranayama on myalgia, headache, psychological stress and respiratory problems in the covid-19 patients in the recovery phase. Biomedicine (India). 2020;40(4):526-30. Available from https://doi.org/10.51248/.v40i4.334 | Wrong study design |
| Pan Hj, Bao Xh, Chen Jy, Feng Y, Kang By, Wang Jx, et al. Respiratory rehabilitation assisted by respiratory trainers in patients with coronavirus disease 2019: an analysis of efficacy. Academic journal of second military medical university. 2021;42(3):255‐60. Available from https://doi.org/10.16781/j.0258-879x.2021.03.0255 | Wrong population |
| Pang W, Yang F, Zhao Y, Dai E, Feng J, Huang Y, et al. Qingjin Yiqi granules for post-COVID-19 condition: A randomized clinical trial. J Evid Based Med. 2022;15(1):30-8. Available from https://doi.org/10.1111/jebm.12465 | Wrong population |
| Paolucci T, Patrizio G, Pietrantonio D, Rapacchiale G, Spacone A, Parruti G, et al. Utility of High Flow Nasal Cannula during Pulmonary Rehabilitation in COVID-19 Patients in Acute Respiratory Failure. Applied Sciences. 2022;12(9):4637-. Available from https://doi.org/10.3390/app12094637 | Wrong population |
| Parker AJ, Humbir A, Tiwary P, Mishra M, Shanmugam M, Bhatia K, et al. Recovery after critical illness in COVID-19 ICU survivors. Br J Anaesth. 2021;126(6):e217-e9. Available from https://doi.org/10.1016/j.bja.2021.03.005 | Wrong study design |
| Patel N, Steinberg C, Patel R, Chomali C, Doulatani G, Lindsay L, Jaywant A. Description and Functional Outcomes of a Novel Interdisciplinary Rehabilitation Program for Hospitalized Patients With COVID-19. Am. J. Phys. Med. Rehabil. 2021;100:1124-32. Available from https://doi.org/10.1097/PHM.0000000000001897 | Wrong study design |
| Paz LES, da Silva Bezerra BJ, de Melo Pereira TM, da Silva WE. Covid-19: The importance of physical therapy in the recovery of workers' health. Revista Brasileira de Medicina do Trabalho. 2021;19(1):94-106. Available from https://doi.org/10.47626/1679-4435-2021-709 | Wrong study design |
| Pehlivan E, Palalı İ, Atan S, Turan D, Çınarka H, Çetinkaya E. The effectiveness of POST-DISCHARGE telerehabilitation practices in COVID-19 patients: Tele-COVID study-randomized controlled trial. Ann Thorac Med. 2022;17(2):110-7. Available from https://doi.org/10.4103/atm.atm_543_21 | Wrong population |
| Pen J, Deslypere JP, Comhaire F. Treating patients with "Long COVID" or "Post COVID Syndrome". Acta Clin. Belg. 2021;76:30-30. | Wrong study design |
| Petraglia F, Chiavilli M, Zaccaria B, Nora M, Mammi P, Ranza E, et al. Rehabilitative treatment of patients with COVID-19 infection: the P.A.R.M.A. evidence based clinical practice protocol. Acta bio-medica : Atenei Parmensis. 2020;91(4):e2020169. Available from https://air.unipr.it/retrieve/handle/11381/2889540/ 223259/Covid%2019%20PARMA%20Protocol.pdf | Wrong study design |
| Piekarski F, Steinbicker AU, Armann JP. The multisystem inflammatory syndrome in children and its association to SARS-CoV-2. Curr Opin Anaesthesiol. 2021;34(4):521-29. Available from https://doi.org/10.1097/ACO.0000000000001024 | Wrong study design |
| Pilloni G, Bikson M, Badran BW, George MS, Kautz SA, Okano AH, et al. Update on the Use of Transcranial Electrical Brain Stimulation to Manage Acute and Chronic COVID-19 Symptoms. Front Hum Neurosci. 2020;14:595567. Available from https://doi.org/10.3389/fnhum.2020.595567 | Wrong study design |
| Piquet V, Luczak C, Seiler F, Monaury J, Martini A, Ward AB, et al. Do Patients With COVID-19 Benefit from Rehabilitation? Functional Outcomes of the First 100 Patients in a COVID-19 Rehabilitation Unit. Archives of physical medicine and rehabilitation, 2021; S0003-9993(21)00134-9. Available from https://doi.org/10.1016/j.apmr.2021.01.069 | Wrong study design |
| Pistarini C, Fiabane E, Houdayer E, Vassallo C, Manera MR, Alemanno F. Cognitive and Emotional Disturbances Due to COVID-19: An Exploratory Study in the Rehabilitation Setting. Front Neurol. 2021;12:8-. Available from https://doi.org/10.3389/fneur.2021.643646 | Wrong study design |
| Polastri M, Nava S, Clini E, Vitacca M, Gosselink R. COVID-19 and pulmonary rehabilitation: preparing for phase three. The European respiratory journal. 2020;55(6). Available from https://doi.org/10.1183/13993003.01822-2020 | Wrong study design |
| Polastri M. Increasing Knowledge on Post-Acute Rehabilitation in COVID-19. Respiration; international review of thoracic diseases. 2021:1-2. Available from https://doi.org/10.1159/000516783 | Wrong study design |
| Polastri M, Costi S. Observational studies of rehabilitation during the COVID-19 pandemic. International Journal of Therapy & Rehabilitation. 2021;28(5):1-3. Available from https://doi.org/10.12968/ijtr.2021.0068 | Wrong study design |
| Poon AN, Akselrod H, Chang A, Adhatamsoontra P, Dobbs J, Morcos GP, et al. Early experiences with a primary care centered long covid-19 clinic. J. Gen. Intern. Med. 2021;36:S386. Available from https://doi.org/10.1007/s11606-021-06830-5 | Wrong study design |
| Prasad A, Elder H, Burke K, Lane N, Ball M, Miller V, et al. A review of outcomes from a novel long-COVID clinic. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.PA620 | Wrong study design |
| Pretorius E, Venter C, Laubscher G.J, Kotze M.J, Moremi K, Oladejo S, Watson L.R, Rajaratnam K, Watson B.W, Kell D.B. Combined triple treatment of fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) can resolve their persistent symptoms. Available from https://doi.org/10.21203/rs.3.rs-1205453/v1 | Wrong study design |
| Priyamvada R, Ranjan R, Chaudhury S. Efficacy of psychological intervention in patients with post-COVID-19 anxiety. Industrial Psychiatry Journal. 2021;30(3):41-4. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8611569/ | Wrong study design |
| Puchner B, Sahanic S, Kirchmair R, Pizzini A, Sonnweber B, Woll E, et al. Beneficial effects of multi-disciplinary rehabilitation in post-acute COVID-19: an observational cohort study. Eur J Phys Rehabil Med. 2021. Available from https://doi.org/10.23736/S1973-9087.21.06549-7 | Wrong study design |
| Puta C, Haunhorst S, Bloch W. Post-acute COVID-19 (“long-COVID”): Prolonged symptoms, possible causes and return to physical fitness (Scoping Review). Sports Orthopaedics and Traumatology 2021. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1433706 | Wrong study design |
| Putrino D, Tabacof L, Tosto-Mancuso J, Wood J, Cortes M, Kontorovich A, et al. Autonomic conditioning therapy reduces fatigue and improves global impression of change in individuals with post-acute COVID-19 syndrome 2021. Available from https://doi.org/10.21203/rs.3.rs-440909/v1 | Wrong study design |
| Rabady S, Altenberger J, Brose M, Denk-Linnert DM, Fertl E, Götzinger F, et al. Guideline S1: Long COVID: Diagnostics and treatment strategies. Wien. Klin. Wochenschr. 2021;133:237-78. Available from https://doi.org/10.1007/s00508-021-01974-0 | Wrong study design |
| Raciti L, Calabro RS. Neurological complications of COVID-19: from pathophysiology to rehabilitation. An overview. Acta bio-medica : Atenei Parmensis 2021;92:e2021317. Available from https://doi.org/10.23750/abm.v92i4.10620 | Wrong intervention |
| Rai DK, Sharma P, Kumar R. Post covid 19 pulmonary fibrosis. Is it reversible? Indian J Tuberc. 2020. Available from https://doi.org/10.1016/j.ijtb.2020.11.003 | Wrong population |
| Rao D, Nomier Y, Ahmed R, Noureldeen A. Retrospective and prospective monitoring in post COVID-19 complications and an approach for vigilance in Post-recovery period. Journal of Advanced Pharmaceutical Technology and Research. 2021;12(2):209-14. Available from https://doi.org/10.4103/japtr.JAPTR_245_20 | Wrong study design |
| Rashid RA, Zgair A, Al-Ani RM. Effect of nasal corticosteroid in the treatment of anosmia due to COVID-19: A randomised double-blind placebo-controlled study. American Journal of Otolaryngology - Head and Neck Medicine and Surgery. 2021;42(5). Available from https://doi.org/10.1016/j.amjoto.2021.103033 | Wrong population |
| Rathi A, Jadhav SB, Shah N. A Randomized Controlled Trial of the Efficacy of Systemic Enzymes and Probiotics in the Resolution of Post-COVID Fatigue. Medicines (Basel, Switzerland). 2021;8(9). Available from https://doi.org/10.3390/medicines8090047 | Wrong population |
| Rawlinson G, Connell L. Out-patient physiotherapy service delivery post COVID-19: opportunity for a re-set and a new normal? Physiotherapy. 2021;111:1-3. Available from https://doi.org/10.1016/j.physio.2021.02.001 | Wrong study design |
| Razaz JM, Nosrati-Oskouie M, Qomi MH, Elham-Kia M, Behzadi-Moghaddam M, Ahadi Z, et al. Nutritional Support for Rehabilitation of Survived COVID-19 Patients: A Review. International Journal of Nutrition Sciences 2021;6:1-5. Available from https://doi.org/10.30476/IJNS.2021.87600.1085 | Wrong study design |
| Reinhardt D. Teleprogram for COVID-19 rehab: Fit again faster with app support. MMW-Fortschritte der Medizin 2021;163:24-26. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/ PMC8413701/pdf/15006_2021_Article_312.pdf | Wrong study design |
| Ren Y, Wang Y, Liu H, Mou F, Yan X, Tang L, Tan D, Zuo G. The Effects of a Comprehensive Rehabilitation Program Involving Traditional Chinese Medicine in Severe and Critical COVID-19 Patients: a Clinical Study2021 2021. Available from https://doi.org/10.21203/rs.3.rs-541774/v1 | Wrong population |
| Rodrigues M, Costa AJ, Santos R, Diogo P, Goncalves E, Barroso D, et al. Inpatient rehabilitation can improve functional outcomes of post-intensive care unit COVID-19 patients-a prospective study. Disabil. Rehabil. 2022:1-11. Available from https://doi.org/10.1080/09638288.2022.2032408 | Wrong study design |
| Rodriguez-Blanco C, Bernal-Utrera C, Anarte-Lazo E, Saavedra-Hernandez M, De-La-Barrera-Aranda E, Serrera-Figallo MA, et al. Breathing exercises versus strength exercises through telerehabilitation in coronavirus disease 2019 patients in the acute phase: A randomized controlled trial. Clin Rehabil. 2021:2692155211061221. Available from https://doi.org/10.1177/02692155211061221 | Wrong population |
| Rolin S, Chakales A, Verduzco-Gutierrez M. Rehabilitation Strategies for Cognitive and Neuropsychiatric Manifestations of COVID-19. Current physical medicine and rehabilitation reports. 2022:1-6. Available from https://doi.org/10.1007/s40141-022-00352-9 | Wrong study design |
| Rooney S, Webster A, Paul L. Systematic Review of Changes and Recovery in Physical Function and Fitness After Severe Acute Respiratory Syndrome–Related Coronavirus Infection: Implications for COVID-19 Rehabilitation. Phys. Ther. 2020;100:1717-29. Available from https://doi.org/10.1093/ptj/pzaa129 | Wrong study design |
| Ros Dopico L, Tung-Chen Y, Pilares Barco M, Munoz Garcia A. Monitoring of the rehabilitation therapy of COVID-19 effort dyspnea. Monitorizacion del tratamiento rehabilitador de la disnea de esfuerzo por COVID-19. 2021;39(5):258-9. Available from https://www.elsevier.es/en-revista-enfermedades-infecciosas-microbiologia-clinica-english-428-articulo-monitoring-rehabilitation-therapy-covid-19-effort-S2529993X21000502 | Wrong study design |
| Rossato MS, Brilli E, Ferri N, Giordano G, Tarantino G. Observational study on the benefit of a nutritional supplement, supporting immune function and energy metabolism, on chronic fatigue associated with the SARS-CoV-2 post-infection progress. Clinical nutrition ESPEN 2021;46:510-18. Available from https://doi.org/10.1016/j.clnesp.2021.08.031 | Wrong population |
| Rossi Ferrario S, Panzeri A, Cerutti P, Sacco D. The Psychological Experience and Intervention in Post-Acute COVID-19 Inpatients. Neuropsychiatr Dis Treat. 2021;17:413-22. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7884934/ | Wrong control group |
| Rota V, Redolfi A, Monteleone S, Arienti C, Falso M. Can COVID-19 result in cognitive dysfunctions? The need for a multidisciplinary approach in rehabilitation for post-COVID-19 people. Eur J Phys Rehabil Med. 2022;58(1):150-1. Available from https://doi.org/10.23736/S1973-9087.21.07013-1 | Wrong study design |
| Routray P, Samal S, Mishra D. Long term morbidity and mortality in covid patients discharged from hospital with or without steroid as discharge medication. Intensive Care Medicine Experimental. 2021;9 | Wrong study design |
| Rozanski GM, Ren I, Sastre C, Iverson B, Tabacof L, Putrino D, Cortes M. Effectiveness of a web-based cognitive rehabilitation program for individuals with long COVID syndrome. PM and R 2021;13:S195 | Wrong study design |
| Ruggeri P, Nair AS, Esquinas A. Comments on "Post severe COVID-19 infection lung damages study. The experience of early three months multidisciplinary follow-up" by De Michele et al. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace. 2022. Available from https://doi.org/10.4081/monaldi.2022.2219 | Wrong study design |
| Rumende CM. Pulmonary Fibrosis Caused by Severe COVID-19 Infection: Discharge May Not Be The End of Treatment. Acta Med. Indones. 2021;53:141-42. Available from https://pubmed.ncbi.nlm.nih.gov/34251340/ | Wrong study design |
| Sá-Caputo DC, Coelho-Oliveira AC, Pessanha-Freitas J, Paineiras-Domingos LL, Lacerda ACR, Mendonça VA, et al. Whole-Body Vibration Exercise: A Possible Intervention in the Management of Post COVID-19 Complications? Applied Sciences. 2021;11(12):5733-. Available from https://doi.org/https://doi.org/10.3390/app11125733 | Wrong population |
| Sakai T, Hoshino C, Yamaguchi R, Hirao M, Nakahara R, Okawa A. Remote rehabilitation for patients with COVID-19. Journal of rehabilitation medicine, 2020;52(9):jrm00095. Available from: https://doi.org/10.2340/16501977-2731 | Wrong population |
| Salvi SS, Ghorpade D, Dhoori S, Dhar R, Dumra H, Chhajed PN, et al. Role of antifibrotic drugs in the management of post-COVID-19 interstitial lung disease: A review of literature and report from an expert working group. Lung India : official organ of Indian Chest Society. 2022;39(2):177-86. Available from https://doi.org/10.4103/lungindia.lungindia_659_21 | Wrong study design |
| Sansone M, Zaami S, Cetta L, Costanzi F, Signore F. Ovotoxicity of smoking and impact on AMH levels: A pilot study. Eur Rev Med Pharmacol Sci. 2021;25(16):5255-60. Available from https://doi.org/10.26355/eurrev_202108_26545 | Wrong population |
| Santana AV, Fontana AD, Pitta F. Pulmonary rehabilitation after COVID-19. J Bras Pneumol. 2021;47(1):e20210034. Available from https://doi.org/10.36416/1806-3756/e20210034 | Wrong study design |
| Santinelli L, Laghi L, Innocenti GP, Pinacchio C, Vassalini P, Celani L, et al. Oral Bacteriotherapy Reduces the Occurrence of Chronic Fatigue in COVID-19 Patients. Frontiers in nutrition. 2021;8:756177. Available from https://doi.org/10.3389/fnut.2021.756177 | Wrong population |
| Sathyamoorthy M, Verduzco-Gutierrez M, Varanasi S, Ward R, Spertus J, Shah S. Enhanced external counterpulsation for management of symptoms associated with long COVID. American Heart Journal Plus: Cardiology Research and Practice. 2022:100105-. Available from https://doi.org/10.1016/j.ahjo.2022.100105 | Wrong study design |
| Saussez S, Vaira LA, Chiesa-Estomba CM, Le Bon SD, Horoi M, Deiana G, et al. Short-term efficacy and safety of oral and nasal corticosteroids in covid-19 patients with olfactory dysfunction: A European multicenter study. Pathogens. 2021;10(6). Available from https://doi.org/10.3390/pathogens10060698 | Wrong population |
| Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. The Lancet Child & adolescent health. 2021;5(6):e22-e3. Available from https://doi.org/10.1016/S2352-4642(21)00124-3 | Wrong intervention |
| Scherlinger M, Pijnenburg L, Chatelus E, Sibilia J, Gottenberg JE, Arnaud L, et al. Effet de la vaccination anti-SARS-CoV-2 sur les symptômes prolongés post-Covid : résultat de l’enquête nationale VAXILONG. Revue du Rhumatisme. 2021;88:A215-A6. Available from https://doi.org/10.1016/j.rhum.2021.10.350 | Wrong study design |
| Schmidt KFR, Gensichen J, Gehrke-Beck S, Kosilek RP, Kühne F, Heintze C, et al. Management of COVID-19 ICU-survivors in primary care: - a narrative review. BMC Fam Pract. 2021;22(1):1-8. Available from https://doi.org/10.1186/s12875-021-01464-2 | Wrong study design |
| Schneeberger T, Jarosch I, Koczulla AR. What can pulmonary rehabilitation accomplish? Dtsch Med Wochenschr. 2020;145(24):1782-5. Available from https://doi.org/10.1055/a-1129-3375 | Wrong study design |
| Sedighimehr N, Fathi J, Hadi N, Rezaeian ZS. Rehabilitation, a necessity in hospitalized and discharged people infected with COVID-19: a narrative review. Phys Ther Rev. 2021. Available from https://doi.org/10.1080/10833196.2021.1899472 | Wrong study design |
| Sepúlveda-Loyola W, Gutiérrez-Espinoza H, Órdenes-Mora J, Araya-Quintanilla F. Práctica basada en evidencia en la rehabilitación post COVID-19: Una mirada desde la Fisioterapia. Fisioterapia 2021. Available from https://www.elsevier.es/es-revista-fisioterapia-146-articulo-practica-basada-evidencia-rehabilitacion-post-covid-19-S0211563821001553 | Wrong study design |
| Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid-19: Summary of NICE, SIGN, and RCGP rapid guideline. The BMJ. 2021;372. Available from https://doi.org/10.1136/bmj.n136 | Wrong study design |
| Shakula AV, Miroshnikov AI. Underwater Vacuum Whirlpool in Medical Rehabilitation of Patients with Postcovid Syndrome. Physical & Rehabilitation Medicine, Medical Rehabilitation 2021;3:159-62. Available from https://doi.org/10.36425/rehab63175 | Wrong study design |
| Shan MX, Tran YM, Vu KT, Eapen BC. Postacute inpatient rehabilitation for COVID-19. BMJ Case Rep. 2020;13(8). Available from https://doi.org/10.1136/bcr-2020-237406 | Wrong study design |
| Sharma P, Goswami SK. Pulmonary Tele-Rehabilitation in Patients (Post Covid-19) With Respiratory Complications: A Randomized Controlled Trial. Indian Journal of Physiotherapy & Occupational Therapy. 2022;16(2):182-9. Available from https://doi.org/10.37506/ijpot.v16i2.18051 | Wrong population |
| Shlapak AA, Zakharova AV, Mekhdieva KR, Nenasheva AV. USE OF PILATES TRAINING AND MYOFASCIAL RELEASE IN REHABILITATION AFTER COVID-19. Human Sport Medicine. 2021;21(3):191-6. | Wrong population |
| Silantyeva ES. The Application of High Intensity and Low Intensity Magnetotherapy in Rehabilitation of Patients with COVID-19: A Randomized Controlled Pilot Study. Physical & Rehabilitation Medicine, Medical Rehabilitation. 2020;2(4):322-8. Available from https://doi.org/10.36425/rehab50236 | Wrong population |
| Simon MA, Luginbuhl RD, Parker R. Reduced incidence of long-COVID symptoms related to administration of COVID-19 vaccines both before COVID-19 diagnosis and up to 12 weeks after. M.A. Simon, Arcadia.io, Burlington, MA, United States; 2021. Available from https://doi.org/10.1101/2021.11.17.21263608 | Wrong study design |
| Singhania SVK, Simon C, Raut A, Parvatkar N. Pulmonary sequelae of moderate-to-severe COVID pneumonia, a 3-month follow-up study. Lung India : official organ of Indian Chest Society 2021;38:397-99. Available from https://doi.org/10.4103/lungindia.lungindia_58_21 | Wrong population |
| Sivan M, Taylor S. NICE guideline on long covid: Research must be done urgently to fill the many gaps in this new "living guideline". The BMJ. 2020;371. Available from https://doi.org/10.1136/bmj.m4938 | Wrong study design |
| Sophie B, Alan KO, Jemina F, Florian L, Sylvain C, Aline S, et al. Virtual reality intervention alleviates dyspnea in patients recovering from COVID pneumonia. A. Dan, Division of Lung Diseases, University Hospital, Geneva Medical School, University of Geneva, Switzerland B. Olaf, Laboratory of Cognitive Neuroscience, Brain Mind Institute, Center for Neuroprosthetics, Faculty of Life Sciences, Ecole Polytechnique Federale de Lausanne, (EPFL), Geneva, Switzerland 2021. Available from https://doi.org/10.1101/2021.10.26.21265510 | Wrong population |
| Soril LJJ, Damant RW, Lam GY, Smith MP, Weatherald J, Bourbeau J, et al. The effectiveness of pulmonary rehabilitation for Post-COVID symptoms: A rapid review of the literature. Respir Med. 2022;195:106782. Available from https://doi.org/10.1016/j.rmed.2022.106782 | Wrong study design |
| Srinivasan V, Kandakurti PK, Alagesan J, Suganthirababu P, Kishore Jebasingh T, Jenifer Augustina S, et al. Efficacy of pursed lip breathing with bhastrika pranayama vs incentive spirometry in rehabilitating post Covid 19 follow up-a randomized control study. Turkish Journal of Physiotherapy and Rehabilitation. 2021;32(3):402-7. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1250736 | Wrong population |
| Stainer A, Faverio P, Busnelli S, Luppi F, Monzani A, Ammatuna F, et al. Pulmonary sequelae in patients with COVID-19: results after 3 months of follow-up. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.PA2535 | Wrong study design |
| Steinberg C, Patel N, Patel R, Jaywant A, Gellhorn A. The Covid Recovery Unit (CRU): An Interdisciplinary Model for Rehabilitation on Acute Care. Arch Phys Med Rehabil. 2021;102(4):e17-e. Available from https://doi.org/10.1016/j.apmr.2021.01.052 | Wrong study design |
| Steurer J. Intranasal steroids seem to have no positive effect for COVID-19 patients with anosmia or hyposmia. Praxis. 2021;110(7):415-6.). | Wrong outcome |
| Stokel-Walker C. On the road to Recovery - The world's biggest covid-19 treatment trial. The BMJ 2021;373. Available from https://doi.org/10.1136/bmj.n1299 | Wrong study design |
| Surendra VU, Mohapatra AK, Roy FA, Sanjai N. A review of pulmonary rehabilitation in patients with covid-19. Critical Reviews in Physical and Rehabilitation Medicine. 2020;32(4):269-83. Available from https://doi.org/10.1615/CritRevPhysRehabilMed.2020036542 | Wrong study design |
| Szczegielniak J, Bogacz K, Majorczyk E, Szczegielniak A, Luniewski J. Post-COVID-19 rehabilitation - a Polish pilot program. Med Pr. 2021. Available from https://doi.org/10.13075/mp.5893.01122 | Wrong study design |
| Tang Y, Jiang J, Shen P, Li M, You H, Liu C, et al. Liuzijue is a promising exercise option for rehabilitating discharged COVID-19 patients. Medicine. 2021;100(6):e24564. Available from https://doi.org/10.1097/MD.0000000000024564 | Wrong control group |
| Tay SS, Neo E, Jr., Tan MM, Tan PL. Post-Critical Care COVID-19 Patient Benefits from a Robotic Patient-Guided Suspension System for Pulmonary Rehabilitation. Ann Acad Med Singapore. 2020;49(6):401-4. Available from https://pubmed.ncbi.nlm.nih.gov/32712640/ | Wrong study design |
| Teitelbaum JGS. An Open-Label, Pilot Trial of HRG80™Red Ginseng in Chronic Fatigue Syndrome, Fibromyalgia, and Post-Viral Fatigue. Pharmaceuticals 2022;15:43-43. Available from https://doi.org/10.3390/ph15010043 | Wrong population |
| Tejerina F, Catalan P, Rodriguez-Grande C, Adan J, Rodriguez-Gonzalez C, Munoz P, et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect Dis. 2022;22(1):211. 2022. Available from https://doi.org/10.1186/s12879-022-07153-4 | Wrong population |
| Toledo C, Vera A, Leija L, Gutierrez J. The Importance of Rehabilitation for COVID-19 Sequelae. Instituto National de Rehabilitación 'Luis Guillermo Ibarra Ibarra, CDMX, Mexico CINVESTAV-IPN, CDMX, Mexico, Mexico: IEEE Computer Society; 2021 2021. Available from https://doi.org/10.1109/GMEPE/PAHCE50215.2021.9434868 | Wrong study design |
| Tomoko S, Chisato H, Reiko Y, Masanobu H, Rui N, Atsushi O. REMOTE REHABILITATION FOR PATIENTS WITH COVID-19. Journal of Rehabilitation Medicine (Stiftelsen Rehabiliteringsinformation) 2020;52:1-8. Available from https://doi.org/10.2340/16501977-2731 | Wrong population |
| Tornero C, Pastor E, Garzando MDM, Orduna J, Forner MJ, Bocigas I, et al. Non-invasive Vagus Nerve Stimulation for COVID-19: Results From a Randomized Controlled Trial (SAVIOR I). Front Neurol. 2022;13:820864. Available from https://doi.org/10.3389/fneur.2022.820864 | Wrong population |
| Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. Available from https://doi.org/10.1371/journal.pone.0240784 | Wrong intervention |
| Tran VT, Perrodeau E, Saldanha J, Pane I, Ravaud P. Efficacy of COVID-19 Vaccination on the Symptoms of Patients With Long COVID: A Target Trial Emulation Using Data From the ComPaRe e-Cohort in France (preprint); 2021. Available from https://doi.org/10.2139/ssrn.3932953 | Wrong study design |
| Tsyganova TN, Balakireva OVK, Kienlein KL, Kapustin AV, Shushardzhan SV. Rationale of the normobaric interval hypoxic training method and the «detensor» method for long-term traction of the spinal column combined application in the complex of rehabilitation measures for post-COVID-19 syndrome. Vestnik Vosstanovitel'noj Mediciny 2021;20:11-15. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1598814 | Wrong study design |
| Turktas H, Oguzulgen IK. Post-COVID-19 pulmonary sequla: longterm follow up and management. COVID-19 sonrasi akciger sekelleri: uzun donem takip ve tedavi. 2020;68(4):419-29. Available from http://www.tuberktoraks.org/managete/fu_folder/2020-04/419-429%20Haluk%20Turktas.pdf | Wrong study design |
| Udina C, Ars J, Morandi A, Vilaro J, Caceres C, Inzitari M. Rehabilitation in adult post-COVID-19 patients in post-acute care with Therapeutic Exercise. The Journal of frailty & aging. 2021;10(3):297-300. Available from https://doi.org/10.14283/jfa.2021.1 | Wrong study design |
| Utrero-Rico A, Ruiz-Ruigomez M, Laguna-Goya R, Arrieta-Ortubay E, Chivite-Lacaba M, Gonzalez-Cuadrado C, et al. A Short Corticosteroid Course Reduces Symptoms and Immunological Alterations Underlying Long-COVID. Biomedicines. 2021;9(11). Available from https://doi.org/10.3390/biomedicines9111540 | Wrong study design |
| Vaira, LA, Hopkins, C, Petrocelli, M, Lechien, JR, Cutrupi, S, Salzano, G, et al. Efficacy of corticosteroid therapy in the treatment of long-lasting olfactory disorders in COVID-19 patients. Rhinology. 2021;59(1):21-25. Available from https://doi.org/10.4193/Rhin20.515 | Wrong population |
| Van Herck M, Goertz Y, Houben-Wilke S, Machado F, Meys R, Delbressine J, et al. Severe fatigue in long COVID - a follow-up study. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.OA1186 | Wrong study design |
| Van Herck M, Goertz YMJ, Houben-Wilke S, Machado FVC, Meys R, Delbressine JM, et al. Severe Fatigue in Long COVID: Web-Based Quantitative Follow-up Study in Members of Online Long COVID Support Groups. J Med Internet Res. 2021;23(9):e30274. Available from https//doi.org/ 10.2196/30274 | Wrong intervention |
| Vandersteen C, Payne M, Dumas LÉ, Cancian É, Plonka A, D’Andrea G, et al. OLFACTORY TRAINING EFFICIENCY IN POST-COVID-19 PERSISTENT OLFACTORY DISORDERS. C. Vandersteen, ENT surgery departement of Institut Universitaire de la Face et du Cou (IUFC), de Valombrose Centre Hospitalier Universitaire (CHU) Université Côte, 31 Avenue, D’Azur (UCA), France2022 2022. Available from https://doi.org/10.1101/2022.02.27.22271572 | Wrong study design |
| Venkatesan P. NICE guideline on long COVID. The Lancet Respiratory medicine. 2021;9(2):129. Available from https://doi.org/10.1016/S2213-2600(21)00031-X | Wrong study design |
| Venturini E, Virgillitto A, Briscese L, Cavicchioli P, Bavera M, Mussini F, et al. Short and medium-term impact of a cardiac rehabilitation (CR) program in COVID-19 patients after acute care hospitalization. Eur Heart J. 2021;42:2678. Available from https://doi.org/10.1093/eurheartj/ehab724.2678 | Wrong study design |
| Vestito L, Mori L, Trompetto C, Bagnasco D, Canevari RF, Ponzano M, et al. Impact of tDCS on persistent COVID-19 olfactory dysfunction: a double-blind sham-controlled study. Journal of neurology, neurosurgery, and psychiatry. 2022. Available from https://doi.org/10.1136/jnnp-2022-329162 | Wrong study design |
| Vetrici MA, Mokmeli S, Bohm AR, Monici M, Sigman SA. Evaluation of Adjunctive Photobiomodulation (PBMT) for COVID-19 Pneumonia via Clinical Status and Pulmonary Severity Indices in a Preliminary Trial. Journal of inflammation research. 2021;14:965-79. Available from https://pubmed.ncbi.nlm.nih.gov/33776469/ | Wrong population |
| Vickory F, Ridgeway K, Falvey J, Houwer B, Gunlikson J, Payne K, et al. Safety, Feasibility, and Outcomes of Frequent, Long-Duration Rehabilitation in an Inpatient Rehabilitation Facility After Prolonged Hospitalization for Severe COVID-19: An Observational Study. Phys Ther. 2021;101(11). Available from https://doi.org/10.1093/ptj/pzab208 | Wrong study design |
| Vieira AGdS, Pinto ACPN, Garcia BMSP, Eid RAC, Mol CG, Nawa RK. Telerehabilitation improves physical function and reduces dyspnoea in people with COVID-19 and post-COVID-19 conditions: a systematic review. J Physiother. 2022. Available from https://doi.org/10.1016/j.jphys.2022.03.011 | Wrong study design |
| Villani G. Effectiveness of rehabilitation in post-COVID compared with post-cardiosurgery patients. A single center experience. European Heart Journal, Supplement. 2021;23:C109. Available from https://doi.org/10.1093/eurjpc/zwab061.385 | Wrong study design |
| Vink M, Vink-Niese A. Could Cognitive Behavioural Therapy Be an Effective Treatment for Long COVID and Post COVID-19 Fatigue Syndrome? Lessons from the Qure Study for Q-Fever Fatigue Syndrome. Healthcare (Basel, Switzerland). 2020;8(4). Available from https://doi.org/10.3390/healthcare8040552 | Wrong population |
| Vishnupriya M, Naveenkumar M, Manjima K, Sooryasree NV, Saranya T, Ramya S, et al. Post-COVID pulmonary fibrosis: Therapeutic efficacy using with mesenchymal stem cells – How the lung heals. Eur. Rev. Med. Pharmacol. Sci. 2021;25:2748-51. Available from https://doi.org/10.26355/eurrev_202103_25438 | Wrong study design |
| Vollbracht C, Kraft K. Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue. Nutrients. 2021;13(4). Available from https://doi.org/10.3390/nu13041154 | Wrong population |
| Wade DT. Rehabilitation after COVID-19: an evidence-based approach. Clin Med. 2020;20(4):359-65. Available from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7385804/ | Wrong study design |
| Wan XY, Meng XZ, Li JC, Gong XL, Liang YQ, Gao SK, et al. Clinical effect of Guanggu Jisheng decoction in treatment of recovery stage coronavirus disease 2019. Academic Journal of Second Military Medical University. 2020;41(7):813-7. Available from https://doi.org/10.16781/j.0258-879x.2020.08.0813 | Wrong study design |
| Wang J, Zhu K, Xue Y, Wen G, Tao L. Research Progress in the Treatment of Complications and Sequelae of COVID-19. Frontiers in medicine 2021;8:757605. Available from https://doi.org/10.3389/fmed.2021.757605 | Wrong study design |
| Wang TJ, Chau B, Lui M, Lam GT, Lin N, Humbert S. Physical Medicine and Rehabilitation and Pulmonary Rehabilitation for COVID-19. Am J Phys Med Rehabil. 2020;99(9):769-74. Available from https://doi.org/10.1097/PHM.0000000000001505 | Wrong study design |
| Wasilewski MB, Cimino SR, Kokorelias KM, Simpson R, Hitzig SL, Robinson L. Providing Rehabilitation to Patients Recovering from COVID-19: A Scoping Review. PM & R : the journal of injury, function, and rehabilitation. 2021. Available from https://doi.org/10.1002/pmrj.12669 | Wrong outcome |
| Wilson C. Vaccines may help clear up long-term covid-19 symptoms. New Scientist. 2021;249(3325):9-. Available from https://doi.org/10.1016/S0262-4079(21)00396-1 | Wrong study design |
| Winship P, Vicary C, Steere N, Lunt D, Musk M, Hill K, et al. Six-minute walk distance of pulmonary rehabilitation participants during COVID-19 restrictions. Respirology. 2021;26:91. Available from https://doi.org/10.1111/resp.14021 | Wrong study design |
| Wolf S, Erdos J. Long COVID care pathways: a systematic review. 2021. Available from https://eprints.aihta.at/1342/ | Wrong study design |
| Workman C, Boles-Ponto L, Kamholz J, Bryant A, Rudroff T. Transcranial Direct Current Stimulation and Post-COVID-19-Fatigue. Brain Stimul. 2021;14(6):1672-3. Available from https://doi.org/10.1016/j.brs.2021.10.268 | Wrong study design |
| World Health Organization. Regional Office for E. [Support for Rehabilitation Self-Management after COVID-19- Related Illness] 2021 | Wrong study design |
| Xavier R, Godoy C, Silva EGE, Iamonti V, Pompeu JE, Toufen C, et al. PULMONARY REHABILITATION IN INDIVIDUALS POS-ACUTE COVID-19 INFECTION: PRELIMINARY RESULTS. Eur Respir J. 2021;58:2-. Available from https://doi.org/10.1183/13993003.congress-2021.OA1188 | Wrong study design |
| Xianyu Y, Wang M, Yue F, Xu X, Yang H, Zhao D, et al. One year follow-up of 18 women who infected COVID-19 while pregnant. J Med Virol. 2022. Available from https://doi.org/10.1002/jmv.27628 | Wrong intervention |
| Yang C-P, Chang C-M, Yang C-C, Pariante CM, Su K-P. Long COVID and Long Chain Fatty Acids (LCFAs): Psychoneuroimmunity implication of omega-3 LCFAs in delayed consequences of COVID-19. Brain Behav Immun. 2022. Available from https://doi.org/10.1016/j.bbi.2022.04.001 | Wrong study design |
| Yavuz V, Ozyurtlu F, Cetin N. Comparison of hydroxychloroquine plus moxifloxacin versus hydroxychloroquine alone on corrected QT interval prolongation in COVID-19 patients. Cor Vasa 2021;65:564-71. Available from https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-1579218 | Wrong population |
| Yelin D, Margalit I. Challenges and Management of Long COVID in Individuals with Hematological Illnesses. Acta Haematol. 2022. Available from https://doi.org/10.1159/000522437 | Wrong study design |
| Yelin D, Moschopoulos CD, Margalit I, Gkrania-Klotsas E, Landi F, Stahl J-P, et al. ESCMID rapid guidelines for assessment and management of long COVID. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2022. Available from https://doi.org/10.1016/j.cmi.2022.02.018 | Wrong study design |
| Yong SJ, Liu S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev. Med. Virol. 2021:e2315. Available from https://doi.org/10.1002/rmv.2315 | Wrong study design |
| Yunliang T, Jian J, Peng S, Moyi L, Huangjun Y, Chongchong L, et al. Liuzijue is a promising exercise option for rehabilitating discharged COVID-19 patients. Medicine. 2021;100(6):1-6. Available from https://doi.org/10.1097/MD.0000000000024564 | Wrong study design |
| Zampogna E, Paneroni M, Belli S, Aliani M, Gandolfo A, Visca D, et al. Pulmonary Rehabilitation in Patients Recovering from COVID-19. Respiration; international review of thoracic diseases. 2021:1-7. Available from https://doi.org/10.1159/000514387 | Wrong study design |
| Zana S, Vecchiato C, Dussin M, Ranieri M, Veronese N. Multicomponent Rehabilitation After COVID-19 for Nursing Home Residents. J Am Med Dir Assoc. 2021. Available from https://doi.org/10.1016/j.jamda.2021.05.001 | Wrong study design |
| Zhen Y. Effect of Aerobics on Rehabilitation Training of New Coronavirus Pneumonia Patients. Basic Clin Pharmacol Toxicol. 2020;127:272‐. Available from https://onlinelibrary.wiley.com/doi/epdf/10.1111/bcpt.13461 | Wrong study design |
| Zheng Y, Zhang X. Effect of Music on Novel Coronavirus Pneumonia Patients' Rehabilitation Training after Recovery. Basic Clin Pharmacol Toxicol. 2020;127:267‐8. | Wrong study design |
| Zhu P, Wang Z, Guo X, Feng Z, Chen C, Zheng A, et al. Pulmonary Rehabilitation Accelerates the Recovery of Pulmonary Function in Patients With COVID-19. Frontiers in cardiovascular medicine. 2021;8:691609. Available from https://doi.org/10.3389/fcvm.2021.691609 | Wrong population |
| Zolotovskaia IA, Shatskaia PR, Davydkin IL, Shavlovskaya OA. Postcovid-19 Asthenic Syndrome. Neurosci Behav Physiol. 2022:1-5. Available from https://doi.org/10.1007/s11055-022-01222-6 | Wrong population |
Relevant review articles
Relevant review articles used to find potential additional primary studies. These review articles have not been assessed for risk of bias.
| Study |
| Alagingi NK. Musculoskeletal physiotherapy strategies in post COVID-19 infection: A narrative review. Journal of Clinical and Diagnostic Research. 2021;15(4):YE06-YE9. Available from https://doi.org/10.7860/JCDR/2021/47155.14706 |
| Andrenelli E, Negrini F, de Sire A, Patrini M, Lazzarini SG, Ceravolo MG, et al. Rehabilitation and COVID-19: update of the rapid living systematic review by Cochrane Rehabilitation Field as of February 28th, 2021. Eur J Phys Rehabil Med. 2021. Available from https://doi.org/10.23736/S1973-9087.21.06995-1 |
| Ceravolo MG, Arienti C, de Sire A, Andrenelli E, Negrini F, Lazzarini SG, et al. Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 rapid living systematic review. Eur J Phys Rehabil Med. 2020;56(5):642-51. Available from https://doi.org/10.23736/S1973-9087.20.06501-6 |
| Cochrane Rehabilitation rapid living systematic reviews. All available from https://rehabilitation.cochrane.org/covid-19/reh-cover-rapid-living-systematic-reviews |
| Demeco A, Marotta N, Barletta M, Pino I, Marinaro C, Petraroli A, et al. Rehabilitation of patients post-COVID-19 infection: a literature review. The Journal of international medical research. 2020;48(8):300060520948382. Available from https://doi.org/10.1177/0300060520948382 |
| Negrini F, de Sire A, Andrenelli E, Lazzarini SG, Patrini M, Ceravolo MG, et al. Rehabilitation and COVID-19: a rapid living systematic review 2020 by Cochrane Rehabilitation Field. Update as of October 31st, 2020. Eur J Phys Rehabil Med. 2021;57(1):166-70. Available from https://doi.org/10.23736/S1973-9087.20.06723-4 |
| Negrini F, de Sire A, Andrenelli E, Lazzarini SG, Patrini M, Ceravolo MG, et al. Rehabilitation and COVID-19: the Cochrane Rehabilitation 2020 rapid living systematic review. Update as of July 31st, 2020. Eur J Phys Rehabil Med. 2020;56(5):652-7. Available from https://doi.org/10.23736/S1973-9087.20.06539-9 |
| Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infectious diseases (London, England). 2021:1-18. Available from https://doi.org/10.1080/23744235.2021.1924397 |
 Statens beredning för medicinsk och social utvärdering
Statens beredning för medicinsk och social utvärdering
 Dela sidan på Facebook
Dela sidan på Facebook
 Dela sidan på LinkedIn
Dela sidan på LinkedIn
 Dela sidan via E-post
Dela sidan via E-post