This publication was published more than 5 years ago. The state of knowledge may have changed.
Molecular Diagnostic Tests for Men at Higher Probability for Prostate Cancer
Summary and Conclusions
SBU’s appraisal of the evidence
Many men, on their own initiative, are tested for the concentration of prostate specific antigen (PSA) in their blood. A high PSA concentration suggests an increased probability of cancer. Men at higher probability often receive further examination by prostate biopsy, an invasive procedure. Since prostate biopsy can lead to complications, unnecessary procedures should be avoided. A result of slightly to moderately raised PSA concentration is difficult to interpret, and uncertainty concerning the necessity of biopsy is common. To avoid unnecessary biopsies, clinicians need new, complementary tests to better determine which men with slightly to moderately raised PSA concentration actually require further investigation.
This report assesses the diagnostic accuracy of three new molecular urine tests (uPCA3, TMPRSS2:ERG, and me-GSTP1) when used to supplement previous PSA testing or rectal examination in investigating prostate cancer. The report does not focus on prostate cancer screening or the benefits of treating prostate cancer. Likewise, we have not assessed the potential importance of the new tests in determining the seriousness of the disease, its prognosis, or the choice of treatment.
- For men with higher probability of prostate cancer, as reflected by PSA testing or rectal examination, diagnostic accuracy increases somewhat by adding the uPCA3 test. The higher the uPCA3 value, the higher the probability of cancer. A uPCA3 result above the threshold value implies a strong suspicion of prostate cancer. In some otherwise difficult-to-assess patients the test would contribute towards earlier biopsy and earlier diagnosis. The uPCA3 test cannot, however, rule out prostate cancer in men having a higher probability of the disease. Accuracy of the uPCA3 test is the same regardless of whether the individual has had a previous biopsy with negative results, or has never had a biopsy.
- The addition of the uPCA3 test to determine which patients should be biopsied involves a higher total cost than direct prostate biopsy of all men with an increased probability of prostate cancer as identified by PSA testing or rectal examination.
- The importance of the uPCA3 test in further investigation and its effects on disease course and mortality should be assessed in clinical studies. To appraise the patient benefits of routine clinical use of uPCA3, better evidence is needed regarding the value of the extra information obtained from the test results in relation to other risk factors, e.g. PSA concentration, age, rectal examination, and the results of previous biopsies.
- The scientific evidence is insufficient to appraise the diagnostic accuracy of TMPRSS2:ERG and me-GSTP1 urine tests. Further studies are needed.
Technology and target group
Prostate cancer is the most common type of cancer among Swedish men, accounting for more than 10 000 new cases annually. Of those diagnosed, approximately one in four die from the disease. Early diagnosis of prostate cancer, at a stage where the disease is not symptomatic or cannot be detected by physical examination, can be beneficial. However, early diagnosis also results in a large number of surgical interventions that provide no benefit for patients and carry a risk for negative effects.
Currently many men, on their own initiative, are examined to determine their probability of prostate cancer while having no real suspicion that they have the disease. Some call this “wild screening”. The individual’s probability of prostate cancer is estimated, e.g. by measuring PSA concentration in the blood and/or by rectal examination. The concentration of PSA is associated with prostate size and increases in prostate cancer. However, since cancer is not the only factor affecting PSA concentration, the PSA test provides uncertain information, especially if the level is slightly or moderately raised. For men with a PSA concentration in the so-called “grey zone”, 3 to 10 nanograms/ml, the decision for further investigation is based on additional factors such as age, life expectancy, heredity, ethnicity, possible symptoms, results of rectal examination, possible results from earlier biopsies, the ratio of free and bound PSA in blood, and the change in PSA concentration over time. Despite the addition of these factors, it is difficult to appraise the risk in many of these men. There is a need to be able to better identify those men at greatest risk for developing clinically significant cancer and who therefore need invasive investigation by prostate biopsy.
Prostate biopsy is currently the reference method for diagnosing prostate cancer. This method has high specificity1, which means that the test seldom indicates a tumour in healthy men. Analysis of the tissue sample from a prostate tumour also provides some information about the possible severity level of the disease and guidance for treatment. However, clinicians are often uncertain whether the identified tumour requires intensive surgical treatment or radiation (accompanied by certain risks and discomfort), or if the tumour is actually harmless. The sensitivity2 of prostate biopsy is limited. Hence, there is a risk of missing tumours. For many patients, prostate biopsies are uncomfortable and painful. The procedure also involves the risk for complications such as bleeding and infection.
A particularly interesting target group for improved testing methods includes men with raised PSA concentration, but where one or more previous biopsies have not identified cancer. Due to the risk that biopsies might miss tumours, these men remain in the risk group for prostate cancer. How to monitor these men through PSA testing and further biopsy is a matter of uncertainty.
Overall, the current voluntary examination of prostate health exposes many patients to both prostate biopsy and surgical removal of the prostate, which ultimately benefits only a few patients. Avoiding unnecessary prostate biopsy is desirable, and better methods are needed to identify which of the tumours detected actually require treatment.
In recent years, several molecular genetic tests for prostate cancer have been developed: urinalysis of gene expression for PCA3 and TMPRSS2:ERG and also GSTP1 methylation levels (me-GSTP1). Several studies have shown that the amounts of these genetic products in urine are associated with the probability of prostate cancer. Testing, which is conducted on urine samples collected in conjunction with rectal examination, is noninvasive, rapid, and not difficult for the patient. The material tested must be handled according to special protocols, but central laboratories analyse the samples. To analyse gene expression for PCA3 in urine, Gen-Probe has developed a test with a recommended threshold value of 35. The probability of prostate cancer is viewed to be greater the higher the results measured in the test. Other tests are not yet commercially available.
It is hoped that these new tests can be used to determine which of the patients with slightly to moderately raised PSA concentration actually need prostate biopsy or repeat biopsy.
Primary questions
- Can the new molecular diagnostic urine tests (uPCA3, TMPRSS2:ERG, and me-GSTP1) identify those men with slightly to moderately raised PSA concentration in blood that actually need further investigation by prostate biopsy?
- Does the addition of the uPCA3 test increase diagnostic accuracy over and above the PSA blood test in men at higher probability of prostate cancer?
- What do the tests cost? Are they cost-effective?
Diagnostic accuracy
All patients participating in the studies that we reviewed had a higher probability of prostate cancer according to the PSA blood test or rectal examination. The reference test has involved at least 8 medium-gauge needle biopsies of the prostate. Most of the included studies have used a commercially available test from Gen-Probe with a threshold value of 35 for positive3 or negative4 responses..
- The uPCA3 test has a sensitivity of 57% (95% CI5 , 54 to 59), compared to prostate biopsy (moderately strong scientific evidence
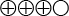 ). This means that the test missed nearly half of the men in whom prostate cancer was identified by biopsy.
). This means that the test missed nearly half of the men in whom prostate cancer was identified by biopsy. - The uPCA3 test has a specificity of 74% (95% CI, 72 to 75), compared to prostate biopsy (moderately strong scientific evidence
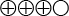 ). This means that 74% of the men in whom biopsy did not show tumours had negative PCA3 results.
). This means that 74% of the men in whom biopsy did not show tumours had negative PCA3 results. - In the studies included in this review, a positive uPCA3 result meant that the probability of disease had increased from 31% to 50% (PPV6). A negative uPCA3 result meant that the probability had decreased from 31% to 21% (when NPV7 is 79%). The change in probability of the disease between before and after the uPCA3 test is small, but could have some importance if the result is positive.
- Based on the included studies, the diagnostic accuracy of the uPCA3 test in men who are re-biopsied after a previously negative biopsy result is the same as in men biopsied for the first time, or where the group is mixed.
- In an indirect comparison between the PSA test alone and the PSA test plus the uPCA3 test, the combination showed a somewhat higher diagnostic accuracy as measured by the area below the ROC curve8. The results apply only to men with higher probability of prostate cancer as indicated by a PSA test or rectal examination.
- The scientific evidence is insufficient (
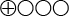 ) to determine the diagnostic accuracy of the TMPRSS2:ERG and the me-GSTP1 urine tests.
) to determine the diagnostic accuracy of the TMPRSS2:ERG and the me-GSTP1 urine tests.
1 Percentage of healthy individuals that the test shows to be healthy.
2 Percentage of sick individuals identified by the test.
3 Positive test results indicate disease.
4 Negative test results indicate absence of disease.
5 Confidence interval (CI).
6 Positive predictive value (PPV). The percentage of those with positive test results that are sick.
7 Negative predictive value (NPV). The percentage of those with negative test results that are healthy.
8 Receiver operating characteristic (ROC), a graphic presentation showing the performance of the test method.
Economic aspects
- The scientific evidence is insufficient (
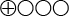 ) to draw reliable conclusions on the cost-effectiveness of the uPCA3 test.
) to draw reliable conclusions on the cost-effectiveness of the uPCA3 test.
In Sweden, the uPCA3 test costs approximately 85% of the cost for a single round of prostate biopsies. Adding the uPCA3 test to determine which patients should be biopsied generates a higher total cost than direct prostate biopsy of the entire risk group.
Four levels are used in grading the strength of the scientific evidence on which conclusions are based:
Strong scientific evidence (![]() ). Based on high or medium quality studies with no factors that weaken the overall assessment.
). Based on high or medium quality studies with no factors that weaken the overall assessment.
Moderately strong scientific evidence (![]() ). Based on high or medium quality studies with isolated factors that weaken the overall assessment.
). Based on high or medium quality studies with isolated factors that weaken the overall assessment.
Limited scientific evidence (![]() ). Based on high or medium quality studies containing factors that weaken the overall assessment.
). Based on high or medium quality studies containing factors that weaken the overall assessment.
Insufficient scientific evidence (![]() ). Scientific evidence is deemed insufficient when scientific findings are absent, the quality of available studies is low, or studies of similar quality present conflicting findings.
). Scientific evidence is deemed insufficient when scientific findings are absent, the quality of available studies is low, or studies of similar quality present conflicting findings.
References
- Nationella prostatacancerregistret (NPCR), 2011.
- Damber JE, Aus G. Prostate cancer. Lancet 2008;371:1710-21.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8.
- Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet 2010;11:725-32.
- Ilic D, O'Connor D, Green S, Wilt TJ. Screening for prostate cancer: an updated Cochrane systematic review. BJU Int 2011;107:882-91.
- Sandblom G, Varenhorst E, Rosell J, Löfman O, Carlsson P. Randomised prostate cancer screening trial: 20 year follow-up. BMJ 2011;342:d1539.
- Sakr WA, Grignon DJ, Crissman JD, Heilbrun LK, Cassin BJ, Pontes JJ, et al. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20-69: an autopsy study of 249 cases. In Vivo 1994;8:439-43.
- Bussemakers MJ, van Bokhoven A, Verhaegh GW, Smit FP, Karthaus HF, Schalken JA, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res 1999;59:5975-9.
- de Kok JB, Verhaegh GW, Roelofs RW, Hessels D, Kiemeney LA, Aalders TW, et al. DD3(PCA3), a very sensitive and specific marker to detect prostate tumors. Cancer Res 2002;62:2695-8.
- Hessels D, Klein Gunnewiek JM, van Oort I, Karthaus HF, van Leenders GJ, van Balken B, et al. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur Urol 2003;44:8-15; discussion 15-6.
- Groskopf J, Aubin SM, Deras IL, Blase A, Bodrug S, Clark C, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem 2006;52:1089-95.
- Taari K, Hotakainen K, Saijonkari M, Grahn R, Leipälä J. PCA3 gene test in diagnosing prostate cancer. Suomen Lääkärilehti 18, 2010.
- Ruiz-Aragón J, Márquez-Peláez S. [Assessment of the PCA3 test for prostate cancer diagnosis: a systematic review and meta-analysis]. Actas Urol Esp 2010;34:346-55.
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 2011;59:61-71.
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310:644-8.
- Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, Rubin MA, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol 2009;56:275-86.
- Harden SV, Sanderson H, Goodman SN, Partin AA, Walsh PC, Epstein JI, et al. Quantitative GSTP1 methylation and the detection of prostate adenocarcinoma in sextant biopsies. J Natl Cancer Inst 2003;95:1634-7.
- Ploussard G, de la Taille A. Urine biomarkers in prostate cancer. Nat Rev Urol 2010;7:101-9.
- Socialstyrelsen. Nationella riktlinjer för bröst-, kolorektal- och prostatacancersjukvård. Beslutsstöd för prioriteringar. Stockholm: Socialstyrelsen; 2007. ISBN 978-91-85483-05-1.
- Thompson IM, Ankerst DP. Prostate-specific antigen in the early detection of prostate cancer. CMAJ 2007;176:1853-8.
- Djavan B, Waldert M, Zlotta A, Dobronski P, Seitz C, Remzi M, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol 2001;166:856-60.
- Borer A, Gilad J, Sikuler E, Riesenberg K, Schlaeffer F, Buskila D. Fatal Clostridium sordellii ischio-rectal abscess with septicaemia complicating ultrasound-guided transrectal prostate biopsy. J Infect 1999;38:128-9.
- Zani EL, Clark OA, Rodrigues Netto N Jr. Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database of Systematic Reviews 2011, Issue 5. Art. No.: CD006576. DOI: 10.1002/14651858.CD006576.pub2.
- Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol 2006;175:1605-12.
- Chun FK, de la Taille A, van Poppel H, Marberger M, Stenzl A, Mulders PF, et al. Prostate cancer gene 3 (PCA3): development and internal validation of a novel biopsy nomogram. Eur Urol 2009;56:659-67.
- Auprich M, Haese A, Walz J, Pummer K, de la Taille A, Graefen M, et al. External validation of urinary PCA3-based nomograms to individually predict prostate biopsy outcome. Eur Urol 2010;58:727-32.
- Laxman B, Morris DS, Yu J, Siddiqui J, Cao J, Mehra R, et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res 2008;68:645-9.
- Auprich M, Chun FK, Ward JF, Pummer K, Babaian R, Augustin H, et al. Critical assessment of preoperative urinary prostate cancer antigen 3 on the accuracy of prostate cancer staging. Eur Urol 2011;59:96-105.
- Hessels D, van Gils MP, van Hooij O, Jannink SA, Witjes JA, Verhaegh GW, et al. Predictive value of PCA3 in urinary sediments in determining clinico-pathological characteristics of prostate cancer. Prostate 2010;70:10-6.
- Nakanishi H, Groskopf J, Fritsche HA, Bhadkamkar V, Blase A, Kumar SV, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol 2008;179:1804-9; discussion 1809-10.
- van Gils MP, Hessels D, Hulsbergen-van de Kaa CA, Witjes JA, Jansen CF, Mulders PF, et al. Detailed analysis of histopathological parameters in radical prostatectomy specimens and PCA3 urine test results. Prostate 2008;68:1215-22.
- Whitman EJ, Groskopf J, Ali A, Chen Y, Blase A, Furusato B, et al. PCA3 score before radical prostatectomy predicts extracapsular extension and tumor volume. J Urol 2008;180:1975-8; discussion 1978-9.
- Vlaeminck-Guillem V, Devonec M, Colombel M, Rodriguez-Lafrasse C, Decaussin-Petrucci M, Ruffion A. Urinary PCA3 score predicts prostate cancer multifocality. J Urol 2011;185:1234-9.
- Tosoian JJ, Loeb S, Kettermann A, Landis P, Elliot DJ, Epstein JI, et al. Accuracy of PCA3 measurement in predicting short-term biopsy progression in an active surveillance program. J Urol 2009;183:534-8.
- Morote J, Rigau M, Garcia M, Mir C, Ballesteros C, Planas J, et al. Behavior of the PCA3 gene in the urine of men with high grade prostatic intraepithelial neoplasia. World J Urol 2010;28:677-80.
- Tinzl M, Marberger M, Horvath S, Chypre C. DD3PCA3 RNA analysis in urine – a new perspective for detecting prostate cancer. Eur Urol 2004;46:182-6; discussion 187.
- Ouyang B, Bracken B, Burke B, Chung E, Liang J, Ho SM. A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-CoA racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer. J Urol 2009;181:2508-13; discussion 2513-4.
- Fradet Y, Saad F, Aprikian A, Dessureault J, Elhilali M, Trudel C, et al. uPM3, a new molecular urine test for the detection of prostate cancer. Urology 2004;64:311-5; discussion 315-6.
- Mearini E, Antognelli C, Del Buono C, Cochetti G, Giannantoni A, Nardelli E, et al. The combination of urine DD3(PCA3) mRNA and PSA mRNA as molecular markers of prostate cancer. Biomarkers 2009;14:235-43.
- Nyberg M, Ulmert D, Lindgren A, Lindström U, Abrahamsson PA, Bjartell A. PCA3 as a diagnostic marker for prostate cancer: A validation study on a Swedish patient population. Scand J Urol Nephrol 2010;44:378-83.
- Rigau M, Morote J, Mir MC, Ballesteros C, Ortega I, Sanchez A, et al. PSGR and PCA3 as biomarkers for the detection of prostate cancer in urine. Prostate 2010;70:1760-7.
- Sokoll LJ, Ellis W, Lange P, Noteboom J, Elliott DJ, Deras IL, et al. A multicenter evaluation of the PCA3 molecular urine test: pre-analytical effects, analytical performance, and diagnostic accuracy. Clin Chim Acta 2008;389:1-6.
- Talesa VN, Antognelli C, Del Buono C, Stracci F, Serva MR, Cottini E, et al. Diagnostic potential in prostate cancer of a panel of urinary molecular tumor markers. Cancer Biomark 2009;5:241-51.
- Galasso F, Giannella R, Bruni P, Giulivo R, Barbini VR, Disanto V, et al. PCA3: a new tool to diagnose prostate cancer (PCa) and a guidance in biopsy decisions. Preliminary report of the UrOP study. Arch Ital Urol Androl 2010;82:5-9.
- Ploussard G, Haese A, van Poppel H, Marberger M, Stenzl A, Mulders PF, et al. The prostate cancer gene 3 (PCA3) urine test in men with previous negative biopsies: does free-to-total prostate-specific antigen ratio influence the performance of the PCA3 score in predicting positive biopsies? BJU Int 2010;106:1143-7.
- Remzi M, Haese A, van Poppel H, de la Taille A, Stenzl A, Hennenlotter J, et al. Follow-up of men with an elevated PCA3 score and a negative biopsy: does an elevated PCA3 score indeed predict the presence of prostate cancer? BJU Int 2010;106:1138-42.
- Shappell SB, Fulmer J, Arguello D, Wright BS, Oppenheimer JR, Putzi MJ. PCA3 urine mRNA testing for prostate carcinoma: patterns of use by community urologists and assay performance in reference laboratory setting. Urology 2009;73:363-8.
- Aubin SM, Reid J, Sarno MJ, Blase A, Aussie J, Rittenhouse H, et al. PCA3 molecular urine test for predicting repeat prostate biopsy outcome in populations at risk: validation in the placebo arm of the dutasteride REDUCE trial. J Urol 2010;184:1947-52.
- de la Taille A, Irani J, Graefen M, Chun F, de Reijke T, Kil P, et al. Clinical evaluation of the PCA3 assay in guiding initial biopsy decisions. J Urol 2011;185:2119-25.
- Marks LS, Fradet Y, Deras IL, Blase A, Mathis J, Aubin SM, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology 2007;69:532-5.
- van Gils MP, Hessels D, van Hooij O, Jannink SA, Peelen WP, Hanssen SL, et al. The time-resolved fluorescence-based PCA3 test on urinary sediments after digital rectal examination; a Dutch multicenter validation of the diagnostic performance. Clin Cancer Res 2007;13:939-43.
- Deras IL, Aubin SM, Blase A, Day JR, Koo S, Partin AW, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol 2008;179:1587-92.
- Haese A, de la Taille A, van Poppel H, Marberger M, Stenzl A, Mulders PF, et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol 2008;54:1081-8.
- Wang R, Chinnaiyan AM, Dunn RL, Wojno KJ, Wei JT. Rational approach to implementation of prostate cancer antigen 3 into clinical care. Cancer 2009;115:3879-86.
- Ochiai A, Okihara K, Kamoi K, Iwata T, Kawauchi A, Miki T, et al. Prostate cancer gene 3 urine assay for prostate cancer in Japanese men undergoing prostate biopsy. Int J Urol 2011;18:200-5.
- Adam A, Engelbrecht MJ, Bornman MS, Manda SO, Moshokoa E, Feilat RA. The role of the PCA3 assay in predicting prostate biopsy outcome in a South African setting. BJU Int 2011:doi: 10.1111/j.1464-410X.2011.10202.x. [Epub ahead of print].
- van Gils MP, Cornel EB, Hessels D, Peelen WP, Witjes JA, Mulders PF, et al. Molecular PCA3 diagnostics on prostatic fluid. Prostate 2007;67:881-7.
- Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31.
- Woodson K, O'Reilly KJ, Hanson JC, Nelson D, Walk EL, Tangrea JA. The usefulness of the detection of GSTP1 methylation in urine as a biomarker in the diagnosis of prostate cancer. J Urol 2008;179:508-11; discussion 511-2.
- Payne SR, Serth J, Schostak M, Kamradt J, Strauss A, Thelen P, et al. DNA methylation biomarkers of prostate cancer: confirmation of candidates and evidence urine is the most sensitive body fluid for non-invasive detection. Prostate 2009;69:1257-69.
- Vener T, Derecho C, Baden J, Wang H, Rajpurohit Y, Skelton J, et al. Development of a multiplexed urine assay for prostate cancer diagnosis. Clin Chem 2008;54:874-82.
- Hessels D, Smit FP, Verhaegh GW, Witjes JA, Cornel EB, Schalken JA. Detection of TMPRSS2-ERG fusion transcripts and prostate cancer antigen 3 in urinary sediments may improve diagnosis of prostate cancer. Clin Cancer Res 2007;13:5103-8.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25.
- Produktblad, 501377SV Rev. B. Gen-Probe.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6.
- Statistiska Centralbyrån (SCB). Lönedatabasen. www.scb.se.
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10.
 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services




