This publication was published more than 5 years ago. The state of knowledge may have changed.
Analysis of Fetal DNA in Maternal Blood: Non-Invasive Fetal Diagnostic Tests for Blood Group and Sex Determination
Summary and conclusions
There is a fetal diagnostic technique available today that involves analysing blood taken from the pregnant woman for the genetic characteristics of her fetus. Non-invasive prenatal diagnosis (NIPD), exploits the presence of cell-free fetal DNA in the blood of the pregnant woman, and often eliminates the need for invasive sampling of e.g. the placenta or amniotic fluid. Small amounts of cell-free fetal DNA are present in the pregnant woman’s plasma during pregnancy but are rapidly eliminated from the mother’s circulation following delivery.
The aim of this evaluation was to establish the accuracy of NIPD in the context of medically indicated fetal blood group and sex determination, and to discuss the health economic and ethical aspects. Sex determination of the fetus in the absence of any medical indication (e.g. for family planning) has not been evaluated. Using the technique for this purpose has been considered unethical in other contexts.
More than 200 scientific publications have been assessed during the evaluation, of which 30 have been assessed in detail. We have focused on three target groups for this technique:
Blood group determination
- Pregnant women immunised against RhD or other blood group antigens
- RhD-negative pregnant women who are not RhD immunised, i.e. screening
Sex determination
- Pregnant women with a medical indication for fetal sex determination as in e.g. X-linked diseases
The use of NIPD for fetal DNA analysis is the subject of active research and development in a variety of clinical fields and in screening for fetal blood groups.
SBU’s appraisal of the evidence
- Some pregnant women have antibodies to (have become immunised against) the blood group of the fetus, which represents a risk to the fetus. The use of NIPD to determine the blood group of the fetus may help to improve the safety of medical care offered to these women and their unborn children.
- Screening for fetal blood group using NIPD, in combination with specific prenatal preventive measures (targeted RhD prophylaxis), could result in fewer RhD-negative pregnant women developing antibodies to RhD. The organisational and health economic consequences of introducing this type of screening have not been established.
- The use of NIPD to determine fetal sex when this is medically justified could improve the safety of medical care by reducing the need for invasive fetal diagnostic tests. The technique is not currently used for this purpose in Sweden.
- There is a lack of published research into the cost-effectiveness of using NIPD to determine fetal sex or blood group.
Summary
Blood group determination
Pregnant women immunised against RhD or other blood group antigens
A pregnant woman who has become immunised against RhD or another blood group, has during current or past pregnancy developed antibodies to a fetal blood group antigen. This is the case in about 1 000 pregnant women in Sweden every year. The woman’s antibodies may destroy the red blood cells of the fetus, which can be life-threatening for the unborn child. In some cases, this has serious consequences for the child, who may need blood transfusion either during pregnancy or after delivery. In rare cases the fetus/child cannot be saved.
NIPD was introduced a few years ago in Sweden for fetal blood group determination in pregnant women immunised against RhD or Rhc. Determination of fetal blood group establishes whether or not the fetus is at risk. If the fetus has a blood group antigen against which the mother has antibodies, the woman needs to be monitored with regular antenatal checks and, possibly, treatment. If the fetus does not have any of these antigens, further tests can be avoided and the parental anxiety in this regard can be alleviated for the remainder of the pregnancy. The use of NIPD can, therefore, help to improve the safety of medical care offered to these women and their unborn children. Used in the way just described, the technique is not thought to raise any significant ethical issues.
RhD-negative pregnant women without RhD immunisation (screening)
NIPD can also be used to screen all RhD-negative pregnant women, enabling the early determination of fetal blood group. About 85 percent of the Swedish population is RhD-positive. The other 15 percent of the population is RhD-negative and lacks the RhD protein, which is the case in about 16 500 pregnant women every year. About 60 percent of these RhD-negative women will carry an RhD-positive baby. In Sweden, RhD prophylaxis is given to these women after delivery, in order to reduce the risk of RhD immunisation.
The introduction of screening to determine fetal RhD status in all RhD-negative women is the subject of debate in Sweden and internationally. It involves a large number of tests and creates new demands on technical platforms. Screening to determine fetal RhD status using NIPD has recently been introduced in Denmark and the Netherlands and is the subject of a study by Stockholm County Council, which is estimated to be completed in 2012.
If NIPD is to be offered in the future to all RhD-negative pregnant women, the objective would be to give RhD prophylaxis during pregnancy (antenatal prophylaxis) only to those carrying an RhD-positive child, in order to reduce the risk of RhD immunisation. The use of NIPD to screen these women would, when combined with targeted RhD prophylaxis, have the potential to reduce the number of new cases of RhD-immunised women. This method could supersede the current practice of antenatal checks and postnatal fetal blood group determination. NIPD used for this purpose offers benefits to women and babies, compared to current practice, and is not considered to be ethically controversial.
Sex determination
NIPD is not currently used in Sweden for medically indicated sex determination but its introduction is planned. The method has the potential to reduce the need for invasive fetal diagnostic tests. If NIPD is used for this purpose, invasive diagnostic tests and late terminations can, in some cases, be avoided. This could reduce medical and psychological risk for the woman.
The use of fetal sex determination to plan families (i.e. sex selection as such) is considered unethical. Basic values of human dignity and respect would be threatened if the technique were to be used in this way. The issue has been considered by the Swedish National Council on Medical Ethics (SMER).
Patient benefit / evidence-graded results
- There is moderately strong scientific evidence that fetal RHD determination by NIPD has a sensitivity and specificity of nearly 99 percent (
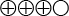 ). These results are largely based on studies of RhD-negative pregnant women who are not RhD-immunised. Those studies that also included pregnant women who have been immunised against RhD showed similar results.
). These results are largely based on studies of RhD-negative pregnant women who are not RhD-immunised. Those studies that also included pregnant women who have been immunised against RhD showed similar results. - The scientific evidence is insufficient to establish the sensitivity and specificity of blood group determination by NIPD in fetal RHC, RHc, RHE and KEL1 (
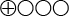 ). It should, however, be noted that these analyses apply to only a few women and, if it is clinically important, the analysis could be repeated as required.
). It should, however, be noted that these analyses apply to only a few women and, if it is clinically important, the analysis could be repeated as required. - There is limited scientific evidence that fetal sex determination by NIPD has a sensitivity and specificity of almost 99 percent (
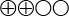 ).
).
Economic aspects
The cost of fetal blood group determination by NIPD was about 2 900 SEK in 2010. The cost of the analysis represented 90 percent of the total cost. NIPD for immunised pregnant women is currently cheaper and has a lower risk of undesirable effects than invasive fetal diagnostic tests.
The health economic consequences of introducing NIPD for the screening of non-RhD-immunised RhD-negative pregnant women are not clear. The cost per analysis within the Swedish healthcare system has not yet been determined.
There is a lack of published research into the cost-effectiveness of using NIPD to determine fetal sex or blood group.
Four levels are used in grading the strength of the scientific evidence on which conclusions are based:
Strong scientific evidence (![]() ). Based on high or medium quality studies with no factors that weaken the overall assessment.
). Based on high or medium quality studies with no factors that weaken the overall assessment.
Moderately strong scientific evidence (![]() ). Based on high or medium quality studies with isolated factors that weaken the overall assessment.
). Based on high or medium quality studies with isolated factors that weaken the overall assessment.
Limited scientific evidence (![]() ). Based on high or medium quality studies having factors that weaken the overall assessment.
). Based on high or medium quality studies having factors that weaken the overall assessment.
Insufficient scientific evidence (![]() ). Scientific evidence is deemed insufficient when scientific findings are absent, the quality of available studies is low, or studies of similar quality present conflicting findings.
). Scientific evidence is deemed insufficient when scientific findings are absent, the quality of available studies is low, or studies of similar quality present conflicting findings.
References
- Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet 1997;350(9076):485-7.
- Lo YM, Hjelm NM, Fidler C, Sargent IL, Murphy MF, Chamberlain PF, et al. Prenatal diagnosis of fetal RhD status by molecular analysis of maternal plasma. N Engl J Med 1998;339(24):1734-8.
- Legler TJ, Liu Z, Mavrou A, Finning K, Hromadnikova I, Galbiati S, et al. Workshop report on the extraction of foetal DNA from maternal plasma. Prenat Diagn 2007;27(9):824-9.
- Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985;230(4732):1350-4.
- Bennett PR, Le Van Kim C, Colin Y, Warwick RM, Chérif-Zahar B, Fisk NM, et al. Prenatal determination of fetal RhD type by DNA amplification. N Engl J Med 1993;329(9):607-10.
- Finning KM, Martin PG, Soothill PW, Avent ND. Prediction of fetal D status from maternal plasma: introduction of a new noninvasive fetal RHD genotyping service. Transfusion 2002;42(8):1079-85.
- Singleton BK, Green CA, Avent ND, Martin PG, Smart E, Daka A, et al. The presence of an RHD pseudogene containing a 37 base pair duplication and a nonsense mutation in africans with the Rh D-negative blood group phenotype. Blood 2000;95(1):12-8.
- Daniels G, van der Schoot CE, Olsson ML. Report of the First International Workshop on molecular blood group genotyping. Vox Sang 2005;88(2):136-42.
- Wright CF, Burton H. The use of cell-free fetal nucleic acids in maternal blood for non-invasive prenatal diagnosis. Hum Reprod Update 2009;15(1):139-51.
- Pilgrim H, Lloyd-Jones M, Rees A. Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation. Health Technol Assess 2009;13(10):iii, ix-xi, 1-103.
- Brojer E, Zupanska B, Guz K, Orziñska A, Kaliñska A. Noninvasive determination of fetal RHD status by examination of cell-free DNA in maternal plasma. Transfusion 2005;45(9):1473-80.
- Cardo L, García BP, Alvarez FV. Non-invasive fetal RHD geno-typing in the first trimester of pregnancy. Clin Chem Lab Med 2010;48(8):1121-6.
- Costa JM, Giovangrandi Y, Ernault P, Lohmann L, Nataf V, El Halali N, et al. Fetal RHD genotyping in maternal serum during the first trimester of pregnancy. Br J Haematol 2002;119(1):255-60.
- Gautier E, Benachi A, Giovangrandi Y, Ernault P, Olivi M, Gaillon T, et al. Fetal RhD genotyping by maternal serum analysis: A two-year experience. Am J Obstet Gynecol 2005;192(3):666-9.
- Grill S, Banzola I, Li Y, Rekhviashvili T, Legler TJ, Müller SP, et al. High throughput non-invasive determination of foetal Rhesus D status using automated extraction of cell-free foetal DNA in maternal plasma and mass spectrometry. Arch Gynecol Obstet 2009;279(4):533-7.
- Randen I, Hauge R, Kjeldsen-Kragh J, Fagerhol MK. Prenatal genotyping of RHD and SRY using maternal blood. Vox Sang 2003;85(4):300-6.
- Rouillac-Le Sciellour C, Sérazin V, Brossard Y, Oudin O, Le Van Kim C, Colin Y, et al. Noninvasive fetal RHD genotyping from maternal plasma. Use of a new developed Free DNA Fetal Kit RhD. Transfus Clin Biol 2007;14(6):572-7.
- Chinen PA, Nardozza LM, Martinhago CD, Camano L, Daher S, Pares DB, et al. Noninvasive determination of fetal rh blood group, D antigen status by cell-free DNA analysis in maternal plasma: experience in a Brazilian population. Am J Perinatol 2010;27(10):759-62.
- Finning K, Martin P, Summers J, Massey E, Poole G, Daniels G. Effect of high throughput RHD typing of fetal DNA in maternal plasma on use of anti-RhD immunoglobulin in RhD negative pregnant women: prospective feasibility study. BMJ 2008;336(7648):816-8.
- Hyland CA, Gardener GJ, Davies H, Ahvenainen M, Flower RL, Irwin D, et al. Evaluation of non-invasive prenatal RHD genotyping of the fetus. Med J Aust 2009;191(1):21-5.
- Minon JM, Gerard C, Senterre JM, Schaaps JP, Foidart JM. Routine fetal RHD genotyping with maternal plasma: a four-year experience in Belgium. Transfusion 2008;48(2):373-81.
- Müller SP, Bartels I, Stein W, Emons G, Gutensohn K, Köhler M, et al. The determination of the fetal D status from maternal plasma for decision making on Rh prophylaxis is feasible. Transfusion 2008;48(11):2292-301.
- Rouillac-Le Sciellour C, Puillandre P, Gillot R, Baulard C, Metral S, Le Van Kim C, et al. Large-scale pre-diagnosis study of fetal RHD genotyping by PCR on plasma DNA from RhD-negative pregnant women. Mol Diagn 2004;8(1):23-31.
- Finning K, Martin P, Summers J, Daniels G. Fetal genotyping for the K (Kell) and Rh C, c, and E blood groups on cell-free fetal DNA in maternal plasma. Transfusion 2007;47(11):2126-33.
- Gutensohn K, Müller SP, Thomann K, Stein W, Suren A, Körtge-Jung S, et al. Diagnostic accuracy of noninvasive polymerase chain reaction testing for the determination of fetal rhesus C, c and E status in early pregnancy. BJOG 2010;117(6):722-9.
- Bustamante-Aragones A, Rodriguez de Alba M, Gonzalez-Gonzalez C, Trujillo-Tiebas MJ, Diego-Alvarez D, Vallespin E, et al. Foetal sex determination in maternal blood from the seventh week of gestation and its role in diagnosing haemophilia in the foetuses of female carriers. Haemophilia 2008;14(3):593-8.
- Costa JM, Benachi A, Gautier E, Jouannic JM, Ernault P, Dumez Y. First-trimester fetal sex determination in maternal serum using real-time PCR. Prenat Diagn 2001;21(12):1070-4.
- Cremonesi L, Galbiati S, Foglieni B, Smid M, Gambini D, Ferrari A, et al. Feasibility study for a microchip-based approach for non-invasive prenatal diagnosis of genetic diseases. Ann N Y Acad Sci 2004;1022:105-12.
- Galbiati S, Smid M, Gambini D, Ferrari A, Restagno G, Viora E, et al. Fetal DNA detection in maternal plasma throughout gestation. Hum Genet 2005;117(2-3):243-8.
- Lapaire O, Volgmann T, Huang D, Hahn S, Holzgreve W, Zhong XY. Maternal smoking: effect on circulating cell-free fetal and total DNA levels in maternal plasma from the second trimester. Obstet Gynecol 2007;110(6):1358-63.
- Nygren AO, Dean J, Jensen TJ, Kruse S, Kwong W, van den Boom D, et al. Quantification of fetal DNA by use of methylation-based DNA discrimination. Clin Chem 2010;56(10):1627-35.
- Picchiassi E, Coata G, Fanetti A, Centra M, Pennacchi L, Di Renzo GC. The best approach for early prediction of fetal gender by using free fetal DNA from maternal plasma. Prenat Diagn 2008;28(6):525-30.
- Ren CC, Miao XH, Cheng H, Chen L, Song WQ. Detection of fetal sex in the peripheral blood of pregnant women. Fetal Diagn Ther 2007;22(5):377-82.
- Sekizawa A, Kondo T, Iwasaki M, Watanabe A, Jimbo M, Saito H, et al. Accuracy of fetal gender determination by analysis of DNA in maternal plasma. Clin Chem 2001;47(10):1856-8.
- Tungwiwat W, Fucharoen S, Fucharoen G, Ratanasiri T, Sanchaisuriya K. Accuracy of fetal gender detection using a conventional nested PCR assay of maternal plasma in daily practice. Aust N Z J Obstet Gynaecol 2008;48(5):501-4.
- Akolekar R, Farkas DH, VanAgtmael AL, Bombard AT, Nicolaides KH. Fetal sex determination using circulating cell-free fetal DNA (ccffDNA) at 11 to 13 weeks of gestation. Prenat Diagn 2010;30(10):918-23.
- Hill M, Finning K, Martin P, Hogg J, Meaney C, Norbury G, et al. Non-invasive prenatal determination of fetal sex: translating research into clinical practice. Clin Genet 2011;80(1):68-75.
- Scheffer PG, van der Schoot CE, Page-Christiaens GC, Bossers B, van Erp F, de Haas M. Reliability of fetal sex determination using maternal plasma. Obstet Gynecol 2010;115(1):117-26.
- Quill E. Blood-matching goes genetic. Science 2008;319(5869):1478-9.
- Crowther CA, Middleton P. Anti-D administration in pregnancy for preventing Rhesus alloimmunisation. Cochrane Database of Systematic Reviews 1999, Issue 2. Art. No.: CD000020. DOI: 10.1002/14651858.CD000020.
- Sundhedsstyrelsen. Anbefalinger for svangreomsorgen. Köpenhamn: Sundhedsstyrelsen; 2009.
- Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci U S A 2008;105(42):16266-71.
- Tong YK, Jin S, Chiu RW, Ding C, Chan KC, Leung TY, et al. Noninvasive prenatal detection of trisomy 21 by an epigenetic-genetic chromosome-dosage approach. Clin Chem 2010;56(1):90-8.
- Chiu RW, Akolekar R, Zheng YW, Leung TY, Sun H, Chan KC, et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. BMJ 2011;342:c7401.
- Tsui NB, Kadir RA, Chan KC, Chi C, Mellars G, Tuddenham EG, et al. Noninvasive prenatal diagnosis of hemophilia by micro-fluidics digital PCR analysis of maternal plasma DNA. Blood 2011;117(13):3684-91.
- Chiu RW, Chan KC, Gao Y, Lau VY, Zheng W, Leung TY, et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A 2008;105(51):20458-63.
- Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Analysis of the size distributions of fetal and maternal cell-free DNA by paired-end sequencing. Clin Chem 2010;56(8):1279-86.
- Statens medicinsk-etiska råd (SMER). Yttrande om etiska frågor kring fosterdiagnostik, 2006-11-06. www.smer.se
- Statens medicinsk-etiska råd (SMER). Fosterdiagnostik – Etisk analys för diagnostik med foster-DNA, september 2011. www.smer.se
- Scheffer P, van der Schoot C, Page-Christiaens G, de Haas M. Noninvasive fetal blood group genotyping of rhesus D, c, E and of K in alloimmunised pregnant women: evaluation of a 7-year clinical experience. BJOG 2011;118(11):1340-8.
- Foster PR, McIntosh RV, Welch AG. Hepatitis C infection from anti-D immunoglobulin. Lancet 1995;346(8971):372.
- Dhallan R, Guo X, Emche S, Damewood M, Bayliss P, Cronin M, et al. A non-invasive test for prenatal diagnosis based on fetal DNA present in maternal blood: a preliminary study. Lancet 2007;369(9560):474-81.
- Bustamante-Aragones A, Pérez-Cerdá C, Pérez B, de Alba MR, Ugarte M, Ramos C. Prenatal diagnosis in maternal plasma of a fetal mutation causing propionic acidemia. Mol Genet Metab 2008;95(1-2):101-3.
- Bustamante-Aragones A, Vallespin E, Rodriguez de Alba M, Trujillo-Tiebas MJ, Gonzalez-Gonzalez C, Diego-Alvarez D, et al. Early noninvasive prenatal detection of a fetal CRB1 mutation causing Leber congenital amaurosis. Mol Vis 2008;14:1388-94.
- Chiu RW, Lau TK, Cheung PT, Gong ZQ, Leung TN, Lo YM. Noninvasive prenatal exclusion of congenital adrenal hyperplasia by maternal plasma analysis: a feasibility study. Clin Chem 2002;48(5):778-80.
- Chiu RW, Lau TK, Leung TN, Chow KC, Chui DH, Lo YM. Prenatal exclusion of beta thalassaemia major by examination of maternal plasma. Lancet 2002;360(9338):998-1000.
- González-González MC, García-Hoyos M, Trujillo MJ, Rodríguez de Alba M, Lorda-Sánchez I, Díaz-Recasens J, et al. Prenatal detection of a cystic fibrosis mutation in fetal DNA from maternal plasma. Prenat Diagn 2002;22(10):946-8.
- Amicucci P, Gennarelli M, Novelli G, Dallapiccola B. Prenatal diagnosis of myotonic dystrophy using fetal DNA obtained from maternal plasma. Clin Chem 2000;46(2):301-2.
- González-González MC, Trujillo MJ, Rodríguez de Alba M, Ramos C. Early Huntington disease prenatal diagnosis by maternal semiquantitative fluorescent-PCR. Neurology 2003;60(7):1214-5.
- Saito H, Sekizawa A, Morimoto T, Suzuki M, Yanaihara T. Prenatal DNA diagnosis of a single-gene disorder from maternal plasma. Lancet 2000;356(9236):1170.
- Bustamante-Aragones A, Garcia-Hoyos M, Rodriguez De Alba M, Gonzalez-Gonzalez C, Lorda-Sanchez I, Diego-Alvarez D, et al. Detection of a paternally inherited fetal mutation in maternal plasma by the use of automated sequencing. Ann N Y Acad Sci 2006;1075:108-17.
- Lo YM, Chan KC, Sun H, Chen EZ, Jiang P, Lun FM, et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci Transl Med 2010;2(61):61ra91.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6.
More on the subject
Scientific Article
Iwarsson E, Jacobsson B, Dagerhamn J, Davidson T, Bernabe E, Heibert Arnlind M. Analysis of cell-free fetal DNA in maternal blood for detection of trisomy 21, 18 and 13 in a general pregnant population and in a high risk population - a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2017;96:7-18.
Read Abstract
 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services




