This publication was published more than 5 years ago. The state of knowledge may have changed.
Peripherally Inserted Central Venous Catheter (PICC)
Summary and conclusions
In severely ill patients, central venous lines (catheter and venous port) are often used to deliver drugs, supply nutrients, or draw blood samples. A peripherally inserted central venous catheter (PICC/PICC line) is a type of central venous line. It is a long, thin catheter constructed of flexible material, often silicone or polyurethane, inserted into a vein in the arm and threaded through the vascular system to the central veins in the chest. Although evidence concerning the method remains unclear, the use of PICC in Swedish health care is increasing because PICC is considered to require fewer resources than conventional central venous catheters.
SBU’s appraisal of the evidence
- Too few studies of sufficiently high quality are available to appraise the functionality of the method, or how often PICC has successfully established central venous access when compared to other central venous lines.
- Likewise, the evidence is insufficient to compare complication risks or patient experiences.The findings suggest a potentially higher risk of deep vein thrombosis, but a potentially lower risk of catheter occlusion, when using PICC.
- Since the medical effects have not been sufficiently studied, the cost-effectiveness of the method cannot be appraised. Studies of high quality are essential to investigate the clinical benefits, risks, and cost-effectiveness of PICC.
Technology and target group
Central venous lines are often used in patients with severe conditions involving, e.g. surgery, anaesthesiology, intensive care, and oncology/haematology. Clinicians use different types of central venous catheters, e.g. tunnelled and non-tunnelled central venous catheters (CVC) or subcutaneous venous ports, depending on the indications and how long the patient will require a central venous line. Insertion of a central venous line requires a physician performing the procedure, mostly in an intensive care unit or an operating theatre.
Insertion of a central venous line carries a risk for serious complications, e.g. pneumothorax, haemorrhage, stroke, and nerve damage. While in use, other complications could also arise, e.g. infection, thrombosis, and catheter occlusion. Serious complications per se, or delayed treatment, can lead to increased suffering for patients and, in some cases, even a fatal outcome. Complications also lead to higher costs, among other things due to the increased need for care.
In recent years, more health care units have started using PICC [1]. A conceivable reason is an increased need for central venous lines. Also, since operating units have a limited capacity for inserting these lines, patients may need to wait for a central venous line. The asserted advantages of using PICCs are that they, compared to other central venous lines, could have fewer serious complications. Also, specially trained nurses can insert the PICC line outside of an operating theatre.
Target groups for PICC are patients in need of treatment involving, e.g. antibiotics, cytostatic drugs (chemotherapy), and nutritional solutions.
Primary questions
- What are the advantages and disadvantages of PICC compared to other central venous lines in terms of early and delayed complications, patient satisfaction, quality of life, functionality, and successful insertion rates in patients needing central venous lines.
- What does treatment cost?
- Is treatment cost-effective?
Patient benefit
- The scientific evidence is insufficient, i.e. studies of sufficiently high quality are not available to draw conclusions on PICC compared to other central venous lines, as regards early and delayed complications, patient satisfaction, quality of life, functionality, and success rates of insertion in patients needing central venous lines (
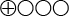 ).
).
This systematic literature review includes 11 studies, 2 of these are of medium quality and serve as a basis for the conclusions. The remaining 9 studies were found to be of low quality. Both of the medium-quality studies are cohort studies that compared PICC to other types of central venous lines. One of these studies, which investigated children and adolescents receiving chemotherapy, reported that PICC presents a higher risk for symptomatic deep vein thrombosis. The results also showed that patients with a PICC were at lower risk for catheter occlusion. The second study, comprised of adult patients discharged from intensive care units for further care at other units, showed that patients with a PICC were at higher risk for deep vein thrombosis. Combined, these findings suggest that PICC could involve a higher risk for deep vein thrombosis, but possibly a lower risk for catheter occlusion. However, additional high-quality studies need to confirm these findings before valid conclusions can be drawn.
Economic aspects
- Since the evidence concerning medical outcomes is insufficient, the cost-effectiveness of using PICCs cannot be appraised.
Four levels are used in grading the strength of the scientific evidence on which conclusions are based:
Strong scientific evidence (![]() ). Based on high or medium quality studies with no factors that weaken the overall assessment.
). Based on high or medium quality studies with no factors that weaken the overall assessment.
Moderately strong scientific evidence (![]() ). Based on high or medium quality studies with isolated factors that weaken the overall assessment.
). Based on high or medium quality studies with isolated factors that weaken the overall assessment.
Limited scientific evidence (![]() ). Based on high or medium quality studies having factors that weaken the overall assessment.
). Based on high or medium quality studies having factors that weaken the overall assessment.
Insufficient scientific evidence (![]() ). Scientific evidence is deemed insufficient when scientific findings are absent, the qual-ity of available studies is low, or studies of similar quality present conflicting findings.
). Scientific evidence is deemed insufficient when scientific findings are absent, the qual-ity of available studies is low, or studies of similar quality present conflicting findings.
References
- Hammarskjöld F, Nielsen N, Rödjer S, Pärsson H, Falkmer U, Malmvall BE. [Peripherally inserted central venous catheter still not evaluated for clinical use. More scientific support is needed according to a literature study]. Läkartidningen 2008;105:1576-80.
- Bhananker SM, Liau DW, Kooner PK, Posner KL, Caplan RA, Domino KB. Liability related to peripheral venous and arterial catheterization: a closed claims analysis. Anesth Analg 2009;109:124-9.
- Svensk Förening för Anestesi och Intensivvård SFAIS:s riktlinjer för centrala venkatetrar. http://www.sfai.se
- Angle JF, Hagspiel KD, Spinosa DJ, Matsumoto AH. Peripherally inserted central catheters. Applied Radiology 1998;27:31-9.
- Seldinger SI. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol 1953;39:368-76.
- Revel-Vilk S, Yacobovich J, Tamary H, Goldstein G, Nemet S, Weintraub M, et al. Risk factors for central venous catheter thrombotic complications in children and adolescents with cancer. Cancer 2010.
- Bonizzoli M, Batacchi S, Cianchi G, Zagli G, Lapi F, Tucci V, et al. Peripherally inserted central venous catheters and central venous catheters related thrombosis in post-critical patients. Intensive Care Med 2010.
- Mollee P, Jones M, Stackelroth J, van Kuilenburg R, Joubert W, Faoagali J, et al. Catheter-associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect 2011;78:26-30.
- Alonso-Echanove J, Edwards JR, Richards MJ, Brennan P, Venezia RA, Keen J, et al. Effect of nurse staffing and antimicrobial-impregnated central venous catheters on the risk for bloodstream infections in intensive care units. Infect Control Hosp Epidemiol 2003;24:916-25.
- Cowl CT, Weinstock JV, Al-Jurf A, Ephgrave K, Murray JA, Dillon K. Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally-inserted central catheters. Clin Nutr 2000;19:237-43.
- Kim HJ, Yun J, Kim KH, Kim SH, Lee SC, Bae SB, et al. Safety and effectiveness of central venous catheterization in patients with cancer: prospective observational study. J Korean Med Sci 2010;25:1748-53.
- Al Raiy B, Fakih MG, Bryan-Nomides N, Hopfner D, Riegel E, Nenninger T, et al. Peripherally inserted central venous catheters in the acute care setting: A safe alternative to high-risk short-term central venous catheters. Am J Infect Control 2010;38:149-53.
- Worth LJ, Seymour JF, Slavin MA. Infective and thrombotic complications of central venous catheters in patients with hematological malignancy: prospective evaluation of nontunneled devices. Support Care Cancer 2009;17:811-8.
- Duerksen DR, Papineau N, Siemens J, Yaffe C. Peripherally inserted central catheters for parenteral nutrition: a comparison with centrally inserted catheters. JPEN J Parenter Enteral Nutr 1999;23:85-9.
- Giuffrida DJ, Bryan-Brown CW, Lumb PD, Kwun KB, Rhoades HM. Central vs peripheral venous catheters in critically ill patients. Chest 1986;90:806-9.
- Raad I, Davis S, Becker M, Hohn D, Houston D, Umphrey J, et al. Low infection rate and long durability of nontunneled silastic catheters. A safe and cost-effective alternative for long-term venous access. Arch Intern Med 1993;153:1791-6.
- Kaplan E L MP, Nonparametric Estimation from Incomplete Observations. 1958.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6.
- Johnson MA, McKenzie L, Tussey S, Jacobs H, Couch C. Portable ultrasound: A cost-effective process improvement tool for PICC placement. Nurs Manage 2009;40:47-50.
- Royer T. Nurse-driven interventional technology. A cost and benefit perspective. J Infus Nurs 2001;24:326-31.
- Horattas MC, Trupiano J, Hopkins S, Pasini D, Martino C, Murty A. Changing concepts in long-term central venous access: catheter selection and cost savings. Am J Infect Control 2001;29:32-40.
- Saber W, Moua T, Williams EC, Verso M, Agnelli G, Couban S, et al. Risk factors of catheter-related thrombosis (CRT) in cancer patients: a patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J Thromb Haemost 2010.
- Sitzia J, Wood N. Patient satisfaction: a review of issues and concepts. Soc Sci Med 1997;45:1829-43.
- Molloy D, Smith LN, Aitchison T. Cytotoxic chemotherapy for incurable colorectal cancer: living with a PICC-line. J Clin Nurs 2008;17:2398-407.
- Polak JF, Anderson D, Hagspiel K, Mungovan J. Peripherally inserted central venous catheters: factors affecting patient satisfaction. AJR Am J Roentgenol 1998;170:1609-11.
- Yamada R, Morita T, Yashiro E, Otani H, Amano K, Tei Y, et al. Patient-reported usefulness of peripherally inserted central venous catheters in terminally ill cancer patients. J Pain Symptom Manage 2010;40:60-6.
- Oakley C, Wright E, Ream E. The experiences of patients and nurses with a nurse-led peripherally inserted central venous catheter line service. Eur J Oncol Nurs 2000;4:207-18.
- Sveriges kommuner och landsting SKL. Förebygg infektioner vid centrala venösa infarter, ISBN 978-91-7164-627-9, 2011.
- Vårdhandboken. http://www.vardhandboken.se
Project group
- Fredrik Hammarskjöld, MD, Consultant, PhD Student, Ryhov County Hospital, Jönköping
- Eva Johansson, RN, Associate Professor, Red Cross University College, Stockholm
- Dag Lundberg, MD, Professor Emeritus, Skåne University Hospital, Lund
- Karin Rydin and Marianne Heibert Arnlind, Project Managers, SBU
SBU staff
- Madelene Lusth Sjöberg, Project Assistant
- Anne Christine Berg, Project Assistant
- Marianne Heibert Arnlind, Health Economist
- Karin Rydin, Literature Searcher
Reviewer
- Urban Nylén, MD, PhD, Karolinska University Hospital, Stockholm
- Anders Själander, MD, Associate Professor, Umeå University, Umeå
More on the subject
Scientific Articles
Johansson E, Hammarskjold F, Lundberg D, Heibert Arnlind M. A survey of the current use of peripherally inserted central venous catheter (PICC) in Swedish oncology departments. Acta Oncol 2013;52:1241-2.
Read Abstract
Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol 2013;52:886-92.
Read Abstract
 Swedish Agency for Health Technology Assessment and Assessment of Social Services
Swedish Agency for Health Technology Assessment and Assessment of Social Services




